69 Radioactive Decay
Learning Objectives
By the end of this section, you will be able to:
- Recognize common modes of radioactive decay
- Identify common particles and energies involved in nuclear decay reactions
- Write and balance nuclear decay equations
- Calculate kinetic parameters for decay processes, including half-life
- Describe common radiometric dating techniques
Following the somewhat serendipitous discovery of radioactivity by Becquerel, many prominent scientists began to investigate this new, intriguing phenomenon. Among them were Marie Curie (the first woman to win a Nobel Prize, and the only person to win two Nobel Prizes in different sciences—chemistry and physics), who was the first to coin the term “radioactivity,” and Ernest Rutherford (of gold foil experiment fame), who investigated and named three of the most common types of radiation. During the beginning of the twentieth century, many radioactive substances were discovered, the properties of radiation were investigated and quantified, and a solid understanding of radiation and nuclear decay was developed.
The spontaneous change of an unstable nuclide into another is radioactive decay. The unstable nuclide is called the parent nuclide; the nuclide that results from the decay is known as the daughter nuclide. The daughter nuclide may be stable, or it may decay itself. The radiation produced during radioactive decay is such that the daughter nuclide lies closer to the band of stability than the parent nuclide, so the location of a nuclide relative to the band of stability can serve as a guide to the kind of decay it will undergo ((Figure)).
Although the radioactive decay of a nucleus is too small to see with the naked eye, we can indirectly view radioactive decay in an environment called a cloud chamber. Click here to learn about cloud chambers and to view an interesting Cloud Chamber Demonstration from the Jefferson Lab.
Types of Radioactive Decay
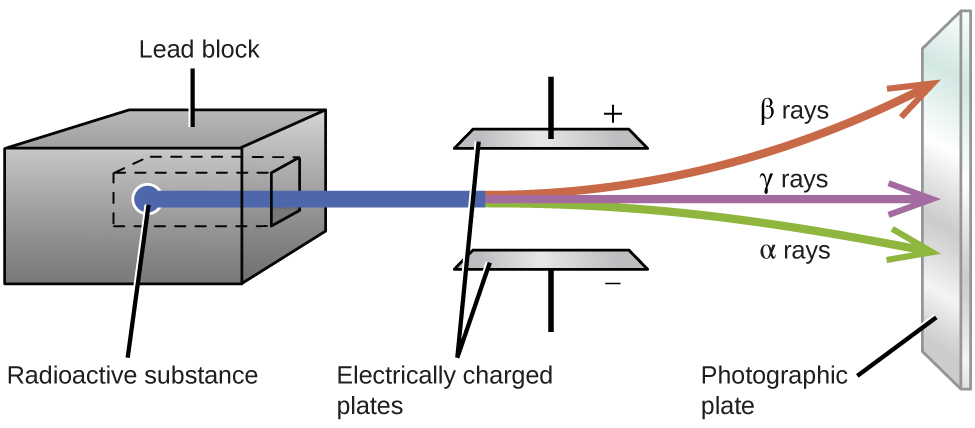
Ernest Rutherford’s experiments involving the interaction of radiation with a magnetic or electric field ((Figure)) helped him determine that one type of radiation consisted of positively charged and relatively massive α particles; a second type was made up of negatively charged and much less massive β particles; and a third was uncharged electromagnetic waves, γ rays. We now know that α particles are high-energy helium nuclei, β particles are high-energy electrons, and γ radiation compose high-energy electromagnetic radiation. We classify different types of radioactive decay by the radiation produced.
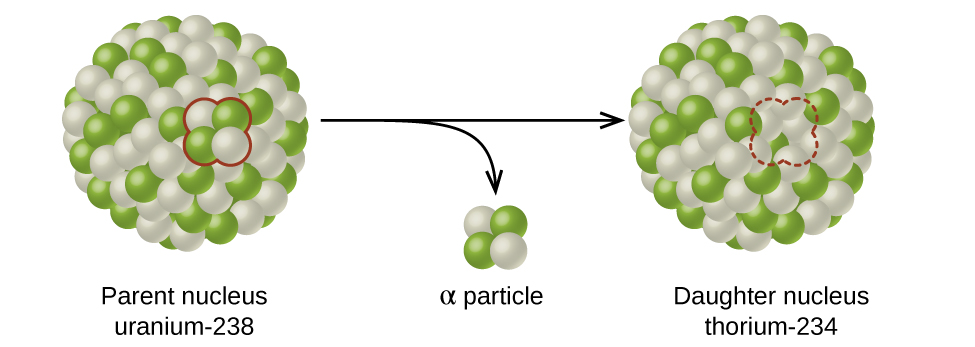
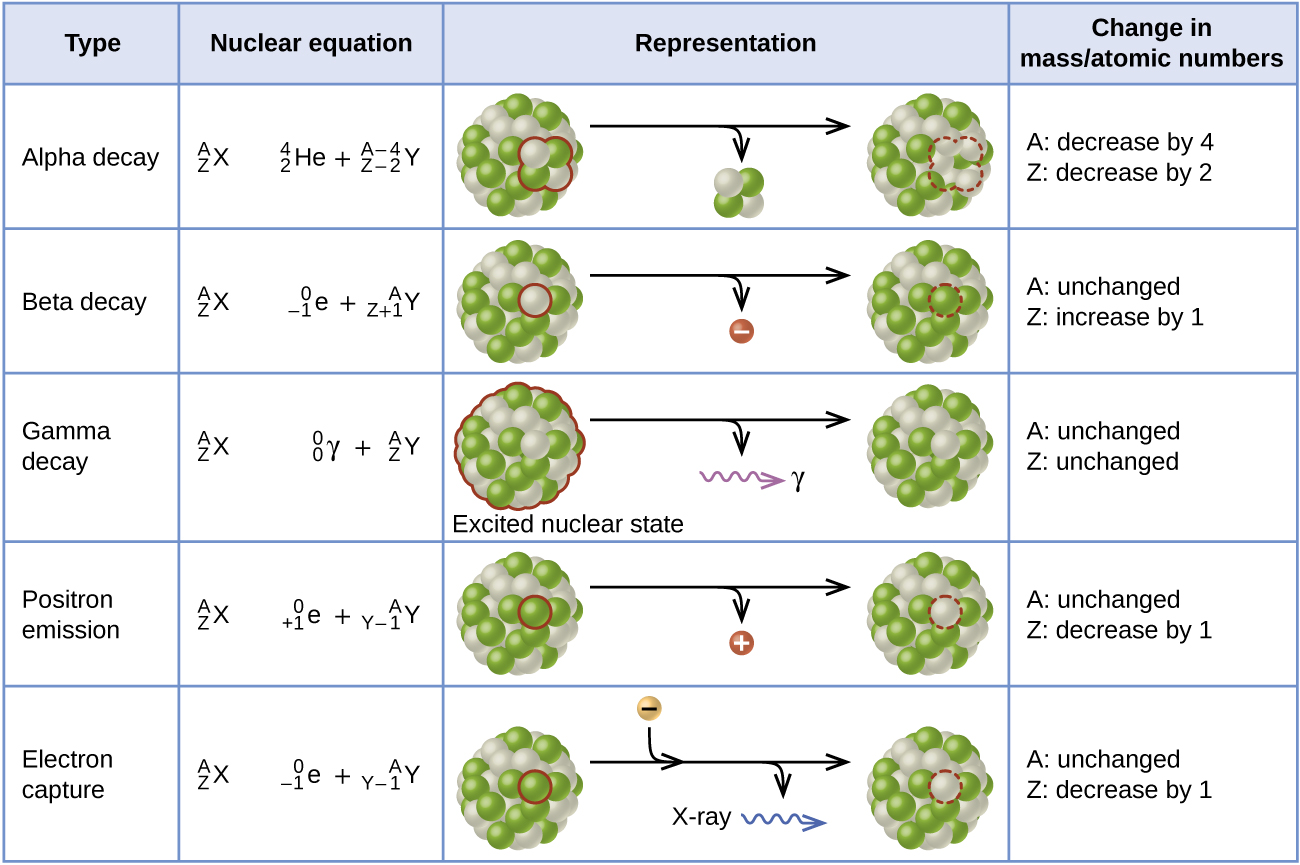
Alpha (α) decay is the emission of an α particle from the nucleus. For example, polonium-210 undergoes α decay:
Alpha decay occurs primarily in heavy nuclei (A > 200, Z > 83). Because the loss of an α particle gives a daughter nuclide with a mass number four units smaller and an atomic number two units smaller than those of the parent nuclide, the daughter nuclide has a larger n:p ratio than the parent nuclide. If the parent nuclide undergoing α decay lies below the band of stability (refer to (Figure)), the daughter nuclide will lie closer to the band.
Beta (β) decay is the emission of an electron from a nucleus. Iodine-131 is an example of a nuclide that undergoes β decay:
Beta decay, which can be thought of as the conversion of a neutron into a proton and a β particle, is observed in nuclides with a large n:p ratio. The beta particle (electron) emitted is from the atomic nucleus and is not one of the electrons surrounding the nucleus. Such nuclei lie above the band of stability. Emission of an electron does not change the mass number of the nuclide but does increase the number of its protons and decrease the number of its neutrons. Consequently, the n:p ratio is decreased, and the daughter nuclide lies closer to the band of stability than did the parent nuclide.
Gamma emission (γ emission) is observed when a nuclide is formed in an excited state and then decays to its ground state with the emission of a γ ray, a quantum of high-energy electromagnetic radiation. The presence of a nucleus in an excited state is often indicated by an asterisk (*). Cobalt-60 emits γ radiation and is used in many applications including cancer treatment:
There is no change in mass number or atomic number during the emission of a γ ray unless the γ emission accompanies one of the other modes of decay.
Positron emission (β+ decay) is the emission of a positron from the nucleus. Oxygen-15 is an example of a nuclide that undergoes positron emission:
Positron emission is observed for nuclides in which the n:p ratio is low. These nuclides lie below the band of stability. Positron decay is the conversion of a proton into a neutron with the emission of a positron. The n:p ratio increases, and the daughter nuclide lies closer to the band of stability than did the parent nuclide.
Electron capture occurs when one of the inner electrons in an atom is captured by the atom’s nucleus. For example, potassium-40 undergoes electron capture:
Electron capture occurs when an inner shell electron combines with a proton and is converted into a neutron. The loss of an inner shell electron leaves a vacancy that will be filled by one of the outer electrons. As the outer electron drops into the vacancy, it will emit energy. In most cases, the energy emitted will be in the form of an X-ray. Like positron emission, electron capture occurs for “proton-rich” nuclei that lie below the band of stability. Electron capture has the same effect on the nucleus as does positron emission: The atomic number is decreased by one and the mass number does not change. This increases the n:p ratio, and the daughter nuclide lies closer to the band of stability than did the parent nuclide. Whether electron capture or positron emission occurs is difficult to predict. The choice is primarily due to kinetic factors, with the one requiring the smaller activation energy being the one more likely to occur.
(Figure) summarizes these types of decay, along with their equations and changes in atomic and mass numbers.
Positron emission tomography (PET) scans use radiation to diagnose and track health conditions and monitor medical treatments by revealing how parts of a patient’s body function ((Figure)). To perform a PET scan, a positron-emitting radioisotope is produced in a cyclotron and then attached to a substance that is used by the part of the body being investigated. This “tagged” compound, or radiotracer, is then put into the patient (injected via IV or breathed in as a gas), and how it is used by the tissue reveals how that organ or other area of the body functions.
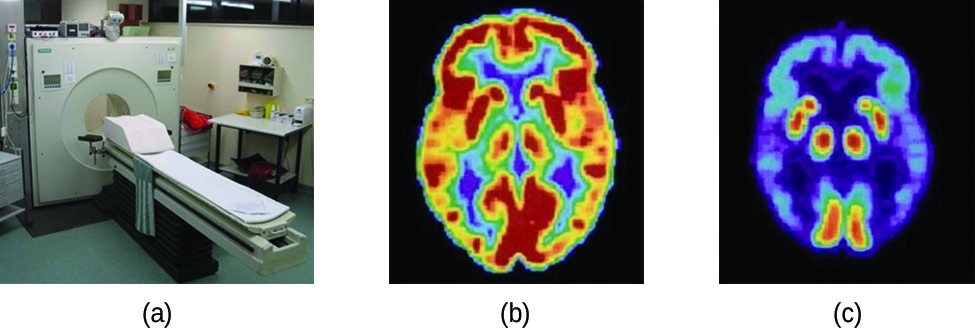
For example, F-18 is produced by proton bombardment of 18O ![]() and incorporated into a glucose analog called fludeoxyglucose (FDG). How FDG is used by the body provides critical diagnostic information; for example, since cancers use glucose differently than normal tissues, FDG can reveal cancers. The 18F emits positrons that interact with nearby electrons, producing a burst of gamma radiation. This energy is detected by the scanner and converted into a detailed, three-dimensional, color image that shows how that part of the patient’s body functions. Different levels of gamma radiation produce different amounts of brightness and colors in the image, which can then be interpreted by a radiologist to reveal what is going on. PET scans can detect heart damage and heart disease, help diagnose Alzheimer’s disease, indicate the part of a brain that is affected by epilepsy, reveal cancer, show what stage it is, and how much it has spread, and whether treatments are effective. Unlike magnetic resonance imaging and X-rays, which only show how something looks, the big advantage of PET scans is that they show how something functions. PET scans are now usually performed in conjunction with a computed tomography scan.
and incorporated into a glucose analog called fludeoxyglucose (FDG). How FDG is used by the body provides critical diagnostic information; for example, since cancers use glucose differently than normal tissues, FDG can reveal cancers. The 18F emits positrons that interact with nearby electrons, producing a burst of gamma radiation. This energy is detected by the scanner and converted into a detailed, three-dimensional, color image that shows how that part of the patient’s body functions. Different levels of gamma radiation produce different amounts of brightness and colors in the image, which can then be interpreted by a radiologist to reveal what is going on. PET scans can detect heart damage and heart disease, help diagnose Alzheimer’s disease, indicate the part of a brain that is affected by epilepsy, reveal cancer, show what stage it is, and how much it has spread, and whether treatments are effective. Unlike magnetic resonance imaging and X-rays, which only show how something looks, the big advantage of PET scans is that they show how something functions. PET scans are now usually performed in conjunction with a computed tomography scan.
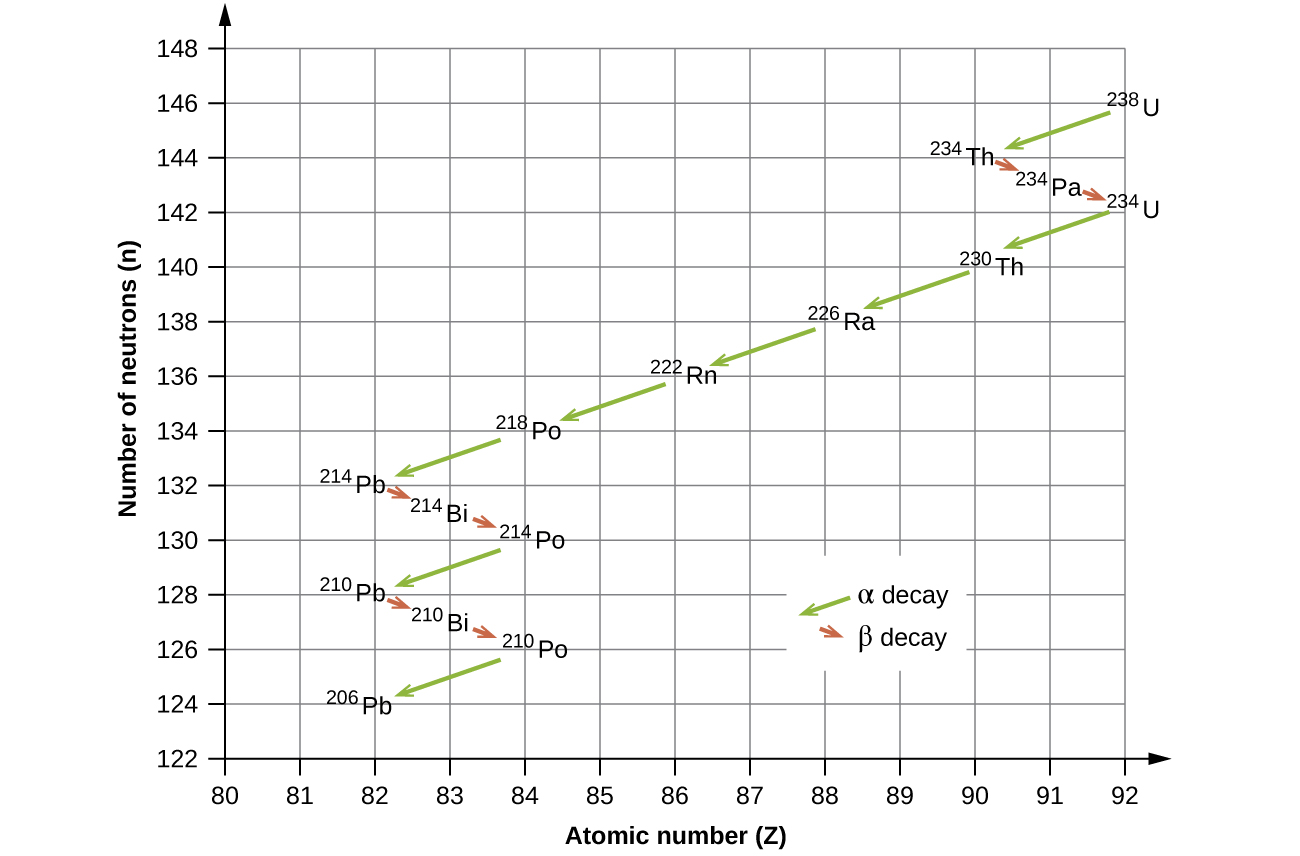
Radioactive Decay Series
The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all the species in one chain constitute a radioactive family, or radioactive decay series. Three of these series include most of the naturally radioactive elements of the periodic table. They are the uranium series, the actinide series, and the thorium series. The neptunium series is a fourth series, which is no longer significant on the earth because of the short half-lives of the species involved. Each series is characterized by a parent (first member) that has a long half-life and a series of daughter nuclides that ultimately lead to a stable end-product—that is, a nuclide on the band of stability ((Figure)). In all three series, the end-product is a stable isotope of lead. The neptunium series, previously thought to terminate with bismuth-209, terminates with thallium-205.
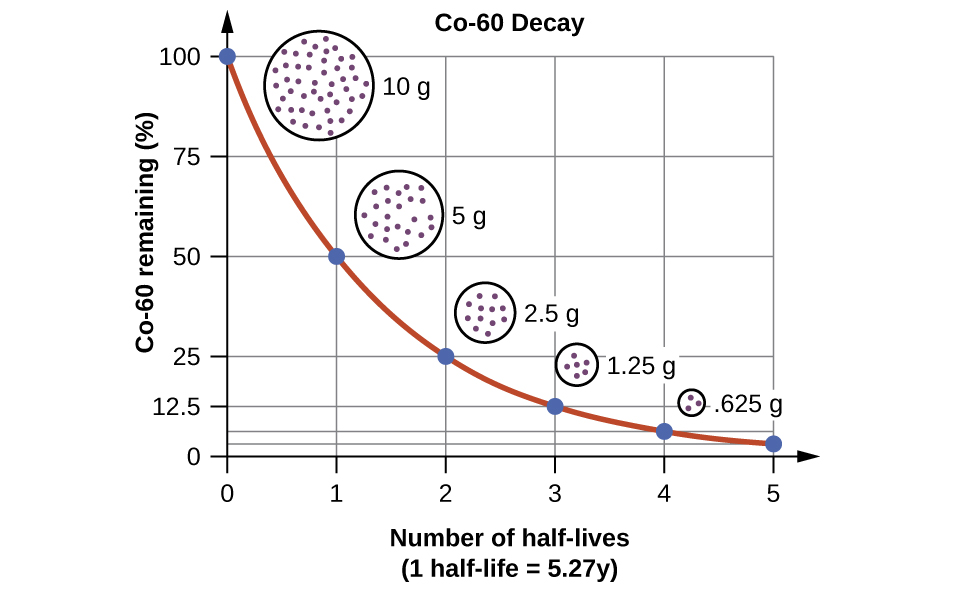
Radioactive Half-Lives
Radioactive decay follows first-order kinetics. Since first-order reactions have already been covered in detail in the kinetics chapter, we will now apply those concepts to nuclear decay reactions. Each radioactive nuclide has a characteristic, constant half-life (t1/2), the time required for half of the atoms in a sample to decay. An isotope’s half-life allows us to determine how long a sample of a useful isotope will be available, and how long a sample of an undesirable or dangerous isotope must be stored before it decays to a low-enough radiation level that is no longer a problem.
For example, cobalt-60, an isotope that emits gamma rays used to treat cancer, has a half-life of 5.27 years ((Figure)). In a given cobalt-60 source, since half of the ![]() nuclei decay every 5.27 years, both the amount of material and the intensity of the radiation emitted is cut in half every 5.27 years. (Note that for a given substance, the intensity of radiation that it produces is directly proportional to the rate of decay of the substance and the amount of the substance.) This is as expected for a process following first-order kinetics. Thus, a cobalt-60 source that is used for cancer treatment must be replaced regularly to continue to be effective.
nuclei decay every 5.27 years, both the amount of material and the intensity of the radiation emitted is cut in half every 5.27 years. (Note that for a given substance, the intensity of radiation that it produces is directly proportional to the rate of decay of the substance and the amount of the substance.) This is as expected for a process following first-order kinetics. Thus, a cobalt-60 source that is used for cancer treatment must be replaced regularly to continue to be effective.
Since nuclear decay follows first-order kinetics, we can adapt the mathematical relationships used for first-order chemical reactions. We generally substitute the number of nuclei, N, for the concentration. If the rate is stated in nuclear decays per second, we refer to it as the activity of the radioactive sample. The rate for radioactive decay is:
decay rate = λN with λ = the decay constant for the particular radioisotope
The decay constant, λ, which is the same as a rate constant discussed in the kinetics chapter. It is possible to express the decay constant in terms of the half-life, t1/2:
The first-order equations relating amount, N, and time are:
where N0 is the initial number of nuclei or moles of the isotope, and Nt is the number of nuclei/moles remaining at time t. (Figure) applies these calculations to find the rates of radioactive decay for specific nuclides.
Rates of Radioactive Decay![]() decays with a half-life of 5.27 years to produce
decays with a half-life of 5.27 years to produce ![]()
(a) What is the decay constant for the radioactive disintegration of cobalt-60?
(b) Calculate the fraction of a sample of the ![]() isotope that will remain after 15 years.
isotope that will remain after 15 years.
(c) How long does it take for a sample of ![]() to disintegrate to the extent that only 2.0% of the original amount remains?
to disintegrate to the extent that only 2.0% of the original amount remains?
Solution (a) The value of the rate constant is given by:
(b) The fraction of ![]() that is left after time t is given by
that is left after time t is given by ![]() Rearranging the first-order relationship Nt = N0e–λt to solve for this ratio yields:
Rearranging the first-order relationship Nt = N0e–λt to solve for this ratio yields:
The fraction of ![]() that will remain after 15.0 years is 0.138. Or put another way, 13.8% of the
that will remain after 15.0 years is 0.138. Or put another way, 13.8% of the ![]() originally present will remain after 15 years.
originally present will remain after 15 years.
(c) 2.00% of the original amount of ![]() is equal to 0.0200
is equal to 0.0200 ![]() N0. Substituting this into the equation for time for first-order kinetics, we have:
N0. Substituting this into the equation for time for first-order kinetics, we have:
Check Your LearningRadon-222, ![]() has a half-life of 3.823 days. How long will it take a sample of radon-222 with a mass of 0.750 g to decay into other elements, leaving only 0.100 g of radon-222?
has a half-life of 3.823 days. How long will it take a sample of radon-222 with a mass of 0.750 g to decay into other elements, leaving only 0.100 g of radon-222?
11.1 days
Because each nuclide has a specific number of nucleons, a particular balance of repulsion and attraction, and its own degree of stability, the half-lives of radioactive nuclides vary widely. For example: the half-life of ![]() is 1.9
is 1.9 ![]() 1019 years;
1019 years; ![]() is 24,000 years;
is 24,000 years; ![]() is 3.82 days; and element-111 (Rg for roentgenium) is 1.5
is 3.82 days; and element-111 (Rg for roentgenium) is 1.5 ![]() 10–3 seconds. The half-lives of a number of radioactive isotopes important to medicine are shown in (Figure), and others are listed in Appendix M.
10–3 seconds. The half-lives of a number of radioactive isotopes important to medicine are shown in (Figure), and others are listed in Appendix M.
| Half-lives of Radioactive Isotopes Important to Medicine | |||
|---|---|---|---|
| Type1 | Decay Mode | Half-Life | Uses |
| F-18 | β+ decay | 110. minutes | PET scans |
| Co-60 | β decay, γ decay | 5.27 years | cancer treatment |
| Tc-99m | γ decay | 8.01 hours | scans of brain, lung, heart, bone |
| I-131 | β decay | 8.02 days | thyroid scans and treatment |
| Tl-201 | electron capture | 73 hours | heart and arteries scans; cardiac stress tests |
Radiometric Dating
Several radioisotopes have half-lives and other properties that make them useful for purposes of “dating” the origin of objects such as archaeological artifacts, formerly living organisms, or geological formations. This process is radiometric dating and has been responsible for many breakthrough scientific discoveries about the geological history of the earth, the evolution of life, and the history of human civilization. We will explore some of the most common types of radioactive dating and how the particular isotopes work for each type.
Radioactive Dating Using Carbon-14
The radioactivity of carbon-14 provides a method for dating objects that were a part of a living organism. This method of radiometric dating, which is also called radiocarbon dating or carbon-14 dating, is accurate for dating carbon-containing substances that are up to about 30,000 years old, and can provide reasonably accurate dates up to a maximum of about 50,000 years old.
Naturally occurring carbon consists of three isotopes: ![]() which constitutes about 99% of the carbon on earth;
which constitutes about 99% of the carbon on earth; ![]() about 1% of the total; and trace amounts of
about 1% of the total; and trace amounts of ![]() Carbon-14 forms in the upper atmosphere by the reaction of nitrogen atoms with neutrons from cosmic rays in space:
Carbon-14 forms in the upper atmosphere by the reaction of nitrogen atoms with neutrons from cosmic rays in space:
All isotopes of carbon react with oxygen to produce CO2 molecules. The ratio of ![]() to
to ![]() depends on the ratio of
depends on the ratio of ![]() to
to ![]() in the atmosphere. The natural abundance of
in the atmosphere. The natural abundance of ![]() in the atmosphere is approximately 1 part per trillion; until recently, this has generally been constant over time, as seen is gas samples found trapped in ice. The incorporation of
in the atmosphere is approximately 1 part per trillion; until recently, this has generally been constant over time, as seen is gas samples found trapped in ice. The incorporation of ![]() and
and ![]() into plants is a regular part of the photosynthesis process, which means that the
into plants is a regular part of the photosynthesis process, which means that the ![]() ratio found in a living plant is the same as the
ratio found in a living plant is the same as the ![]() ratio in the atmosphere. But when the plant dies, it no longer traps carbon through photosynthesis. Because
ratio in the atmosphere. But when the plant dies, it no longer traps carbon through photosynthesis. Because ![]() is a stable isotope and does not undergo radioactive decay, its concentration in the plant does not change. However, carbon-14 decays by β emission with a half-life of 5730 years:
is a stable isotope and does not undergo radioactive decay, its concentration in the plant does not change. However, carbon-14 decays by β emission with a half-life of 5730 years:
Thus, the ![]() ratio gradually decreases after the plant dies. The decrease in the ratio with time provides a measure of the time that has elapsed since the death of the plant (or other organism that ate the plant). (Figure) visually depicts this process.
ratio gradually decreases after the plant dies. The decrease in the ratio with time provides a measure of the time that has elapsed since the death of the plant (or other organism that ate the plant). (Figure) visually depicts this process.
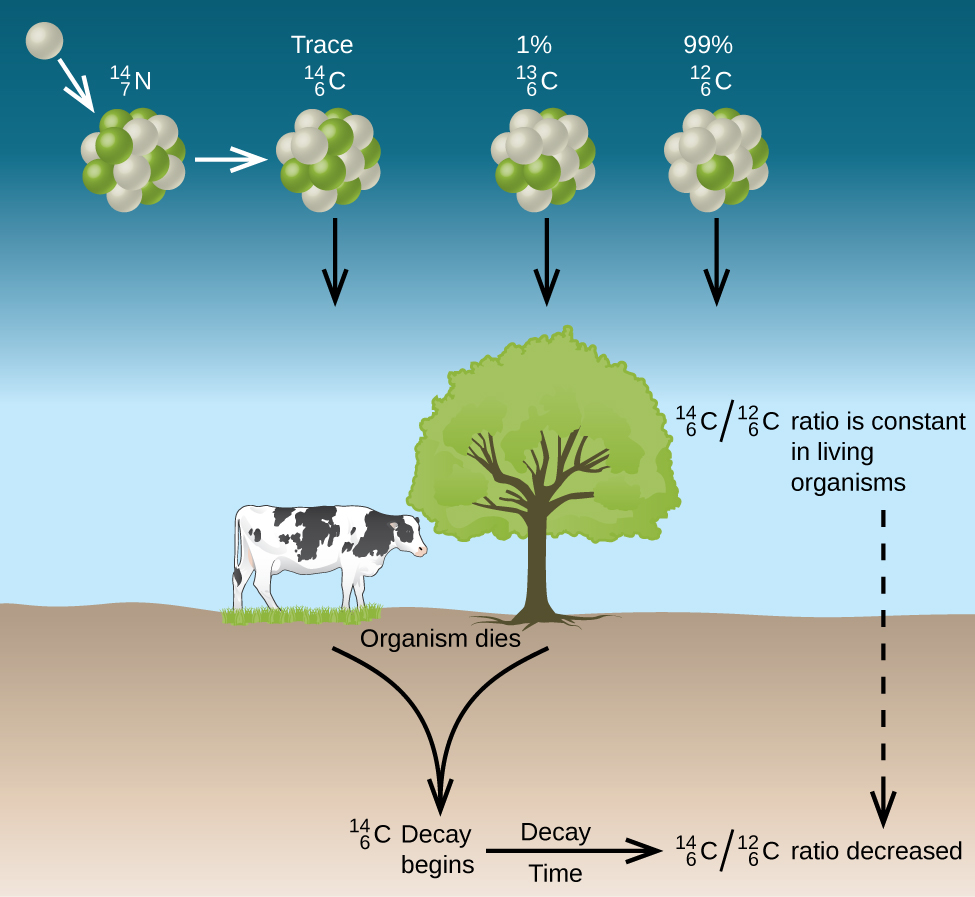
For example, with the half-life of ![]() being 5730 years, if the
being 5730 years, if the ![]() ratio in a wooden object found in an archaeological dig is half what it is in a living tree, this indicates that the wooden object is 5730 years old. Highly accurate determinations of
ratio in a wooden object found in an archaeological dig is half what it is in a living tree, this indicates that the wooden object is 5730 years old. Highly accurate determinations of ![]() ratios can be obtained from very small samples (as little as a milligram) by the use of a mass spectrometer.
ratios can be obtained from very small samples (as little as a milligram) by the use of a mass spectrometer.
Visit this website to perform simulations of radiometric dating.
Radiocarbon Dating A tiny piece of paper (produced from formerly living plant matter) taken from the Dead Sea Scrolls has an activity of 10.8 disintegrations per minute per gram of carbon. If the initial C-14 activity was 13.6 disintegrations/min/g of C, estimate the age of the Dead Sea Scrolls.
Solution The rate of decay (number of disintegrations/minute/gram of carbon) is proportional to the amount of radioactive C-14 left in the paper, so we can substitute the rates for the amounts, N, in the relationship:
where the subscript 0 represents the time when the plants were cut to make the paper, and the subscript t represents the current time.
The decay constant can be determined from the half-life of C-14, 5730 years:
Substituting and solving, we have:
Therefore, the Dead Sea Scrolls are approximately 1900 years old ((Figure)).

Check Your Learning More accurate dates of the reigns of ancient Egyptian pharaohs have been determined recently using plants that were preserved in their tombs. Samples of seeds and plant matter from King Tutankhamun’s tomb have a C-14 decay rate of 9.07 disintegrations/min/g of C. How long ago did King Tut’s reign come to an end?
about 3350 years ago, or approximately 1340 BC
There have been some significant, well-documented changes to the ![]() ratio. The accuracy of a straightforward application of this technique depends on the
ratio. The accuracy of a straightforward application of this technique depends on the ![]() ratio in a living plant being the same now as it was in an earlier era, but this is not always valid. Due to the increasing accumulation of CO2 molecules (largely
ratio in a living plant being the same now as it was in an earlier era, but this is not always valid. Due to the increasing accumulation of CO2 molecules (largely ![]() in the atmosphere caused by combustion of fossil fuels (in which essentially all of the
in the atmosphere caused by combustion of fossil fuels (in which essentially all of the ![]() has decayed), the ratio of
has decayed), the ratio of ![]() in the atmosphere may be changing. This manmade increase in
in the atmosphere may be changing. This manmade increase in ![]() in the atmosphere causes the
in the atmosphere causes the ![]() ratio to decrease, and this in turn affects the ratio in currently living organisms on the earth. Fortunately, however, we can use other data, such as tree dating via examination of annual growth rings, to calculate correction factors. With these correction factors, accurate dates can be determined. In general, radioactive dating only works for about 10 half-lives; therefore, the limit for carbon-14 dating is about 57,000 years.
ratio to decrease, and this in turn affects the ratio in currently living organisms on the earth. Fortunately, however, we can use other data, such as tree dating via examination of annual growth rings, to calculate correction factors. With these correction factors, accurate dates can be determined. In general, radioactive dating only works for about 10 half-lives; therefore, the limit for carbon-14 dating is about 57,000 years.
Radioactive Dating Using Nuclides Other than Carbon-14
Radioactive dating can also use other radioactive nuclides with longer half-lives to date older events. For example, uranium-238 (which decays in a series of steps into lead-206) can be used for establishing the age of rocks (and the approximate age of the oldest rocks on earth). Since U-238 has a half-life of 4.5 billion years, it takes that amount of time for half of the original U-238 to decay into Pb-206. In a sample of rock that does not contain appreciable amounts of Pb-208, the most abundant isotope of lead, we can assume that lead was not present when the rock was formed. Therefore, by measuring and analyzing the ratio of U-238:Pb-206, we can determine the age of the rock. This assumes that all of the lead-206 present came from the decay of uranium-238. If there is additional lead-206 present, which is indicated by the presence of other lead isotopes in the sample, it is necessary to make an adjustment. Potassium-argon dating uses a similar method. K-40 decays by positron emission and electron capture to form Ar-40 with a half-life of 1.25 billion years. If a rock sample is crushed and the amount of Ar-40 gas that escapes is measured, determination of the Ar-40:K-40 ratio yields the age of the rock. Other methods, such as rubidium-strontium dating (Rb-87 decays into Sr-87 with a half-life of 48.8 billion years), operate on the same principle. To estimate the lower limit for the earth’s age, scientists determine the age of various rocks and minerals, making the assumption that the earth is older than the oldest rocks and minerals in its crust. As of 2014, the oldest known rocks on earth are the Jack Hills zircons from Australia, found by uranium-lead dating to be almost 4.4 billion years old.
Radioactive Dating of Rocks An igneous rock contains 9.58 ![]() 10–5 g of U-238 and 2.51
10–5 g of U-238 and 2.51 ![]() 10–5 g of Pb-206, and much, much smaller amounts of Pb-208. Determine the approximate time at which the rock formed.
10–5 g of Pb-206, and much, much smaller amounts of Pb-208. Determine the approximate time at which the rock formed.
Solution The sample of rock contains very little Pb-208, the most common isotope of lead, so we can safely assume that all the Pb-206 in the rock was produced by the radioactive decay of U-238. When the rock formed, it contained all of the U-238 currently in it, plus some U-238 that has since undergone radioactive decay.
The amount of U-238 currently in the rock is:
Because when one mole of U-238 decays, it produces one mole of Pb-206, the amount of U-238 that has undergone radioactive decay since the rock was formed is:
The total amount of U-238 originally present in the rock is therefore:
The amount of time that has passed since the formation of the rock is given by:
with N0 representing the original amount of U-238 and Nt representing the present amount of U-238.
U-238 decays into Pb-206 with a half-life of 4.5 ![]() 109 y, so the decay constant λ is:
109 y, so the decay constant λ is:
Substituting and solving, we have:
Therefore, the rock is approximately 1.7 billion years old.
Check Your Learning A sample of rock contains 6.14 ![]() 10–4 g of Rb-87 and 3.51
10–4 g of Rb-87 and 3.51 ![]() 10–5 g of Sr-87. Calculate the age of the rock. (The half-life of the β decay of Rb-87 is 4.7
10–5 g of Sr-87. Calculate the age of the rock. (The half-life of the β decay of Rb-87 is 4.7 ![]() 1010 y.)
1010 y.)
3.7 ![]() 109 y
109 y
Key Concepts and Summary
Nuclei that have unstable n:p ratios undergo spontaneous radioactive decay. The most common types of radioactivity are α decay, β decay, γ emission, positron emission, and electron capture. Nuclear reactions also often involve γ rays, and some nuclei decay by electron capture. Each of these modes of decay leads to the formation of a new nucleus with a more stable n:p ratio. Some substances undergo radioactive decay series, proceeding through multiple decays before ending in a stable isotope. All nuclear decay processes follow first-order kinetics, and each radioisotope has its own characteristic half-life, the time that is required for half of its atoms to decay. Because of the large differences in stability among nuclides, there is a very wide range of half-lives of radioactive substances. Many of these substances have found useful applications in medical diagnosis and treatment, determining the age of archaeological and geological objects, and more.
Key Equations
- decay rate = λN
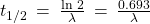
Chemistry End of Chapter Exercises
1. What are the types of radiation emitted by the nuclei of radioactive elements?
α (helium nuclei), β (electrons), β+ (positrons), and η (neutrons) may be emitted from a radioactive element, all of which are particles; γ rays also may be emitted.
2. What changes occur to the atomic number and mass of a nucleus during each of the following decay scenarios?
(a) an α particle is emitted
(b) a β particle is emitted
(c) γ radiation is emitted
(d) a positron is emitted
(e) an electron is captured
3. What is the change in the nucleus that results from the following decay scenarios?
(a) emission of a β particle
(b) emission of a β+ particle
(c) capture of an electron
(a) conversion of a neutron to a proton: ![]() (b) conversion of a proton to a neutron; the positron has the same mass as an electron and the same magnitude of positive charge as the electron has negative charge; when the n:p ratio of a nucleus is too low, a proton is converted into a neutron with the emission of a positron:
(b) conversion of a proton to a neutron; the positron has the same mass as an electron and the same magnitude of positive charge as the electron has negative charge; when the n:p ratio of a nucleus is too low, a proton is converted into a neutron with the emission of a positron: ![]() (c) In a proton-rich nucleus, an inner atomic electron can be absorbed. In simplest form, this changes a proton into a neutron:
(c) In a proton-rich nucleus, an inner atomic electron can be absorbed. In simplest form, this changes a proton into a neutron: ![]()
4. Many nuclides with atomic numbers greater than 83 decay by processes such as electron emission. Explain the observation that the emissions from these unstable nuclides also normally include α particles.
5. Why is electron capture accompanied by the emission of an X-ray?
The electron pulled into the nucleus was most likely found in the 1s orbital. As an electron falls from a higher energy level to replace it, the difference in the energy of the replacement electron in its two energy levels is given off as an X-ray.
6. Explain, in terms of (Figure), how unstable heavy nuclides (atomic number > 83) may decompose to form nuclides of greater stability (a) if they are below the band of stability and (b) if they are above the band of stability.
7. Which of the following nuclei is most likely to decay by positron emission? Explain your choice.
(a) chromium-53
(b) manganese-51
(c) iron-59
Manganese-51 is most likely to decay by positron emission. The n:p ratio for Cr-53 is ![]() = 1.21; for Mn-51, it is
= 1.21; for Mn-51, it is ![]() = 1.04; for Fe-59, it is
= 1.04; for Fe-59, it is ![]() = 1.27. Positron decay occurs when the n:p ratio is low. Mn-51 has the lowest n:p ratio and therefore is most likely to decay by positron emission. Besides,
= 1.27. Positron decay occurs when the n:p ratio is low. Mn-51 has the lowest n:p ratio and therefore is most likely to decay by positron emission. Besides, ![]() is a stable isotope, and
is a stable isotope, and ![]() decays by beta emission.
decays by beta emission.
8. The following nuclei do not lie in the band of stability. How would they be expected to decay? Explain your answer.
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
(e) ![]()
9. The following nuclei do not lie in the band of stability. How would they be expected to decay?
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
(e) ![]()
(a) β decay; (b) α decay; (c) positron emission; (d) β decay; (e) α decay
10. Predict by what mode(s) of spontaneous radioactive decay each of the following unstable isotopes might proceed:
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
(e) 18F
(f) 129Ba
(g) 237Pu
11. Write a nuclear reaction for each step in the formation of ![]() from
from ![]() which proceeds by a series of decay reactions involving the step-wise emission of α, β, β, α, α, α particles, in that order.
which proceeds by a series of decay reactions involving the step-wise emission of α, β, β, α, α, α particles, in that order.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
12. Write a nuclear reaction for each step in the formation of ![]() from
from ![]() which proceeds by a series of decay reactions involving the step-wise emission of α, α, α, α, β, β, α particles, in that order.
which proceeds by a series of decay reactions involving the step-wise emission of α, α, α, α, β, β, α particles, in that order.
13. Define the term half-life and illustrate it with an example.
14. Half-life is the time required for half the atoms in a sample to decay. Example (answers may vary): For C-14, the half-life is 5770 years. A 10-g sample of C-14 would contain 5 g of C-14 after 5770 years; a 0.20-g sample of C-14 would contain 0.10 g after 5770 years.
15. A 1.00 ![]() 10–6-g sample of nobelium,
10–6-g sample of nobelium, ![]() has a half-life of 55 seconds after it is formed. What is the percentage of
has a half-life of 55 seconds after it is formed. What is the percentage of ![]() remaining at the following times?
remaining at the following times?
(a) 5.0 min after it forms
(b) 1.0 h after it forms
16. 239Pu is a nuclear waste byproduct with a half-life of 24,000 y. What fraction of the 239Pu present today will be present in 1000 y?
![]() or 97.3%
or 97.3%
17. The isotope 208Tl undergoes β decay with a half-life of 3.1 min.
(a) What isotope is produced by the decay?
(b) How long will it take for 99.0% of a sample of pure 208Tl to decay?
(c) What percentage of a sample of pure 208Tl remains un-decayed after 1.0 h?
18. If 1.000 g of ![]() produces 0.0001 mL of the gas
produces 0.0001 mL of the gas ![]() at STP (standard temperature and pressure) in 24 h, what is the half-life of 226Ra in years?
at STP (standard temperature and pressure) in 24 h, what is the half-life of 226Ra in years?
2 ![]() 103 y
103 y
19. The isotope ![]() is one of the extremely hazardous species in the residues from nuclear power generation. The strontium in a 0.500-g sample diminishes to 0.393 g in 10.0 y. Calculate the half-life.
is one of the extremely hazardous species in the residues from nuclear power generation. The strontium in a 0.500-g sample diminishes to 0.393 g in 10.0 y. Calculate the half-life.
20. Technetium-99 is often used for assessing heart, liver, and lung damage because certain technetium compounds are absorbed by damaged tissues. It has a half-life of 6.0 h. Calculate the rate constant for the decay of ![]()
0.12 h–1
21. What is the age of mummified primate skin that contains 8.25% of the original quantity of 14C?
22. A sample of rock was found to contain 8.23 mg of rubidium-87 and 0.47 mg of strontium-87.
(a) Calculate the age of the rock if the half-life of the decay of rubidium by β emission is 4.7 ![]() 1010 y.
1010 y.
(b) If some ![]() was initially present in the rock, would the rock be younger, older, or the same age as the age calculated in (a)? Explain your answer.
was initially present in the rock, would the rock be younger, older, or the same age as the age calculated in (a)? Explain your answer.
(a) 3.8 billion years; (b) The rock would be younger than the age calculated in part (a). If Sr was originally in the rock, the amount produced by radioactive decay would equal the present amount minus the initial amount. As this amount would be smaller than the amount used to calculate the age of the rock and the age is proportional to the amount of Sr, the rock would be younger.
23. A laboratory investigation shows that a sample of uranium ore contains 5.37 mg of ![]() and 2.52 mg of
and 2.52 mg of ![]() Calculate the age of the ore. The half-life of
Calculate the age of the ore. The half-life of ![]() is 4.5
is 4.5 ![]() 109 yr.
109 yr.
24. Plutonium was detected in trace amounts in natural uranium deposits by Glenn Seaborg and his associates in 1941. They proposed that the source of this 239Pu was the capture of neutrons by 238U nuclei. Why is this plutonium not likely to have been trapped at the time the solar system formed 4.7 ![]() 109 years ago?
109 years ago?
c = 0; This shows that no Pu-239 could remain since the formation of the earth. Consequently, the plutonium now present could not have been formed with the uranium.
25. A ![]() atom (mass = 7.0169 amu) decays into a
atom (mass = 7.0169 amu) decays into a ![]() atom (mass = 7.0160 amu) by electron capture. How much energy (in millions of electron volts, MeV) is produced by this reaction?
atom (mass = 7.0160 amu) by electron capture. How much energy (in millions of electron volts, MeV) is produced by this reaction?
26. A ![]() atom (mass = 8.0246 amu) decays into a
atom (mass = 8.0246 amu) decays into a ![]() atom (mass = 8.0053 amu) by loss of a β+ particle (mass = 0.00055 amu) or by electron capture. How much energy (in millions of electron volts) is produced by this reaction?
atom (mass = 8.0053 amu) by loss of a β+ particle (mass = 0.00055 amu) or by electron capture. How much energy (in millions of electron volts) is produced by this reaction?
17.5 MeV
27. Isotopes such as 26Al (half-life: 7.2 ![]() 105 years) are believed to have been present in our solar system as it formed, but have since decayed and are now called extinct nuclides.
105 years) are believed to have been present in our solar system as it formed, but have since decayed and are now called extinct nuclides.
(a) 26Al decays by β+ emission or electron capture. Write the equations for these two nuclear transformations.
(b) The earth was formed about 4.7 ![]() 109 (4.7 billion) years ago. How old was the earth when 99.999999% of the 26Al originally present had decayed?
109 (4.7 billion) years ago. How old was the earth when 99.999999% of the 26Al originally present had decayed?
28. Write a balanced equation for each of the following nuclear reactions:
(a) bismuth-212 decays into polonium-212
(b) beryllium-8 and a positron are produced by the decay of an unstable nucleus
(c) neptunium-239 forms from the reaction of uranium-238 with a neutron and then spontaneously converts into plutonium-239
(d) strontium-90 decays into yttrium-90
(a) ![]() (b)
(b) ![]() (c)
(c) ![]()
![]() (d)
(d) ![]()
29. Write a balanced equation for each of the following nuclear reactions:
(a) mercury-180 decays into platinum-176
(b) zirconium-90 and an electron are produced by the decay of an unstable nucleus
(c) thorium-232 decays and produces an alpha particle and a radium-228 nucleus, which decays into actinium-228 by beta decay
(d) neon-19 decays into fluorine-19
Footnotes
- 1The “m” in Tc-99m stands for “metastable,” indicating that this is an unstable, high-energy state of Tc-99. Metastable isotopes emit γ radiation to rid themselves of excess energy and become (more) stable.
Glossary
- alpha (α) decay
- loss of an alpha particle during radioactive decay
- beta (β) decay
- breakdown of a neutron into a proton, which remains in the nucleus, and an electron, which is emitted as a beta particle
- daughter nuclide
- nuclide produced by the radioactive decay of another nuclide; may be stable or may decay further
- electron capture
- combination of a core electron with a proton to yield a neutron within the nucleus
- gamma (γ) emission
- decay of an excited-state nuclide accompanied by emission of a gamma ray
- half-life (t1/2)
- time required for half of the atoms in a radioactive sample to decay
- parent nuclide
- unstable nuclide that changes spontaneously into another (daughter) nuclide
- positron emission
- (also, β+ decay) conversion of a proton into a neutron, which remains in the nucleus, and a positron, which is emitted
- radioactive decay
- spontaneous decay of an unstable nuclide into another nuclide
- radioactive decay series
- chains of successive disintegrations (radioactive decays) that ultimately lead to a stable end-product
- radiocarbon dating
- highly accurate means of dating objects 30,000–50,000 years old that were derived from once-living matter; achieved by calculating the ratio of
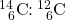 in the object vs. the ratio of
in the object vs. the ratio of 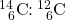 in the present-day atmosphere
in the present-day atmosphere
- radiometric dating
- use of radioisotopes and their properties to date the formation of objects such as archeological artifacts, formerly living organisms, or geological formations

