40 Classifying Chemical Reactions
Learning Objectives
By the end of this section, you will be able to:
- Define and recognize two common types of chemical reactions (acid-base, and oxidation-reduction)
- Identify common acids and bases
Acid-Base Reactions
An acid-base reaction is one in which a hydrogen ion, H+, is transferred from one chemical species to another. Such reactions are of central importance to numerous natural and technological processes, ranging from the chemical transformations that take place within cells and the lakes and oceans, to the industrial-scale production of fertilizers, pharmaceuticals, and other substances essential to society. The subject of acid-base chemistry, therefore, is worthy of thorough discussion, and a full chapter is devoted to this topic later in the text.
For purposes of this brief introduction, we will consider only the more common types of acid-base reactions that take place in aqueous solutions. In this context, an acid is a substance that will dissolve in water to yield hydronium ions, H3O+. As an example, consider the equation shown here:
The process represented by this equation confirms that hydrogen chloride is an acid. When dissolved in water, H3O+ ions are produced by a chemical reaction in which H+ ions are transferred from HCl molecules to H2O molecules ((Figure)).
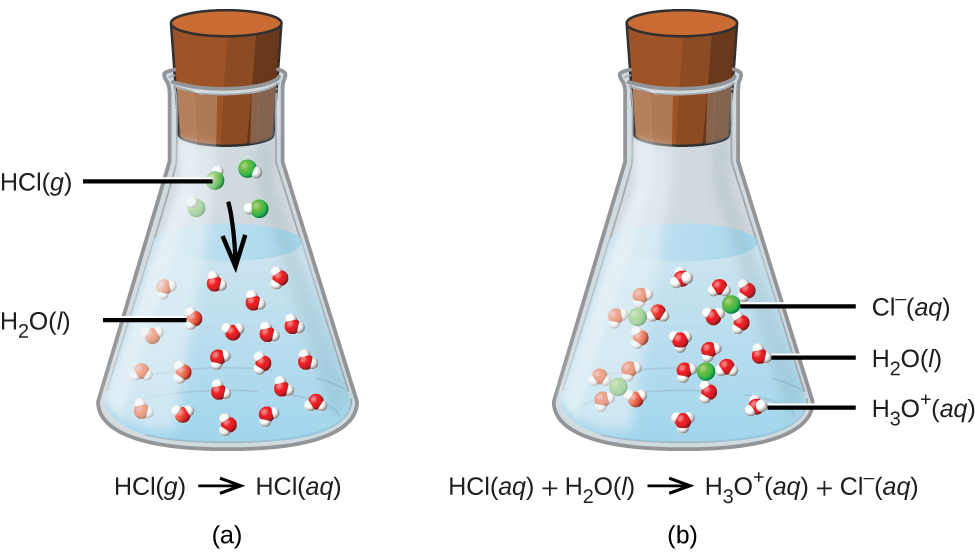
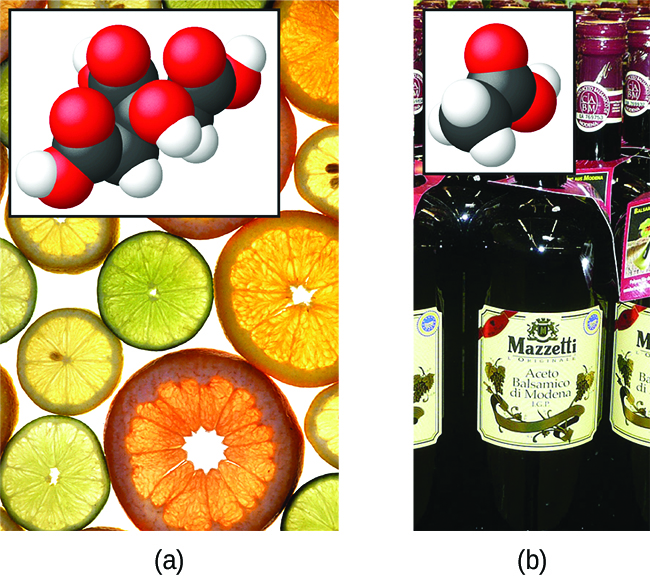
The nature of HCl is such that its reaction with water as just described is essentially 100% efficient: Virtually every HCl molecule that dissolves in water will undergo this reaction. Acids that completely react in this fashion are called strong acids, and HCl is one among just a handful of common acid compounds that are classified as strong ((Figure)). A far greater number of compounds behave as weak acids and only partially react with water, leaving a large majority of dissolved molecules in their original form and generating a relatively small amount of hydronium ions. Weak acids are commonly encountered in nature, being the substances partly responsible for the tangy taste of citrus fruits, the stinging sensation of insect bites, and the unpleasant smells associated with body odor. A familiar example of a weak acid is acetic acid, the main ingredient in food vinegars:
When dissolved in water under typical conditions, only about 1% of acetic acid molecules are present in the ionized form, ![]() ((Figure)). (The use of a double-arrow in the equation above denotes the partial reaction aspect of this process, a concept addressed fully in the chapters on chemical equilibrium.)
((Figure)). (The use of a double-arrow in the equation above denotes the partial reaction aspect of this process, a concept addressed fully in the chapters on chemical equilibrium.)
| Common Strong Acids | ||
|---|---|---|
| Compound Formula | Name in Aqueous Solution | |
| HBr | hydrobromic acid | |
| HCl | hydrochloric acid | |
| HI | hydroiodic acid | |
| HNO3 | nitric acid | |
| HClO4 | perchloric acid | |
| H2SO4 | sulfuric acid | |
A base is a substance that will dissolve in water to yield hydroxide ions, OH−. The most common bases are ionic compounds composed of alkali or alkaline earth metal cations (groups 1 and 2) combined with the hydroxide ion—for example, NaOH and Ca(OH)2. Unlike the acid compounds discussed previously, these compounds do not react chemically with water; instead they dissolve and dissociate, releasing hydroxide ions directly into the solution. For example, KOH and Ba(OH)2 dissolve in water and dissociate completely to produce cations (K+ and Ba2+, respectively) and hydroxide ions, OH−. These bases, along with other hydroxides that completely dissociate in water, are considered strong bases.
Consider as an example the dissolution of lye (sodium hydroxide) in water:
This equation confirms that sodium hydroxide is a base. When dissolved in water, NaOH dissociates to yield Na+ and OH− ions. This is also true for any other ionic compound containing hydroxide ions. Since the dissociation process is essentially complete when ionic compounds dissolve in water under typical conditions, NaOH and other ionic hydroxides are all classified as strong bases.
Unlike ionic hydroxides, some compounds produce hydroxide ions when dissolved by chemically reacting with water molecules. In all cases, these compounds react only partially and so are classified as weak bases. These types of compounds are also abundant in nature and important commodities in various technologies. For example, global production of the weak base ammonia is typically well over 100 metric tons annually, being widely used as an agricultural fertilizer, a raw material for chemical synthesis of other compounds, and an active ingredient in household cleaners ((Figure)). When dissolved in water, ammonia reacts partially to yield hydroxide ions, as shown here:
This is, by definition, an acid-base reaction, in this case involving the transfer of H+ ions from water molecules to ammonia molecules. Under typical conditions, only about 1% of the dissolved ammonia is present as ![]() ions.
ions.

A neutralization reaction is a specific type of acid-base reaction in which the reactants are an acid and a base (but not water), and the products are often a salt and water
To illustrate a neutralization reaction, consider what happens when a typical antacid such as milk of magnesia (an aqueous suspension of solid Mg(OH)2) is ingested to ease symptoms associated with excess stomach acid (HCl):
Note that in addition to water, this reaction produces a salt, magnesium chloride.
Writing Equations for Acid-Base Reactions Write balanced chemical equations for the acid-base reactions described here:
(a) the weak acid hydrogen hypochlorite reacts with water
(b) a solution of barium hydroxide is neutralized with a solution of nitric acid
Solution (a) The two reactants are provided, HOCl and H2O. Since the substance is reported to be an acid, its reaction with water will involve the transfer of H+ from HOCl to H2O to generate hydronium ions, H3O+ and hypochlorite ions, OCl−.
A double-arrow is appropriate in this equation because it indicates the HOCl is a weak acid that has not reacted completely.
(b) The two reactants are provided, Ba(OH)2 and HNO3. Since this is a neutralization reaction, the two products will be water and a salt composed of the cation of the ionic hydroxide (Ba2+) and the anion generated when the acid transfers its hydrogen ion ![]()
Check Your Learning Write the net ionic equation representing the neutralization of any strong acid with an ionic hydroxide. (Hint: Consider the ions produced when a strong acid is dissolved in water.)
![]()
Our stomachs contain a solution of roughly 0.03 M HCl, which helps us digest the food we eat. The burning sensation associated with heartburn is a result of the acid of the stomach leaking through the muscular valve at the top of the stomach into the lower reaches of the esophagus. The lining of the esophagus is not protected from the corrosive effects of stomach acid the way the lining of the stomach is, and the results can be very painful. When we have heartburn, it feels better if we reduce the excess acid in the esophagus by taking an antacid. As you may have guessed, antacids are bases. One of the most common antacids is calcium carbonate, CaCO3. The reaction,
not only neutralizes stomach acid, it also produces CO2(g), which may result in a satisfying belch.
Milk of Magnesia is a suspension of the sparingly soluble base magnesium hydroxide, Mg(OH)2. It works according to the reaction:
The hydroxide ions generated in this equilibrium then go on to react with the hydronium ions from the stomach acid, so that:
This reaction does not produce carbon dioxide, but magnesium-containing antacids can have a laxative effect. Several antacids have aluminum hydroxide, Al(OH)3, as an active ingredient. The aluminum hydroxide tends to cause constipation, and some antacids use aluminum hydroxide in concert with magnesium hydroxide to balance the side effects of the two substances.
Examples of acid-base chemistry are abundant in the culinary world. One example is the use of baking soda, or sodium bicarbonate in baking. NaHCO3 is a base. When it reacts with an acid such as lemon juice, buttermilk, or sour cream in a batter, bubbles of carbon dioxide gas are formed from decomposition of the resulting carbonic acid, and the batter “rises.” Baking powder is a combination of sodium bicarbonate, and one or more acid salts that react when the two chemicals come in contact with water in the batter.
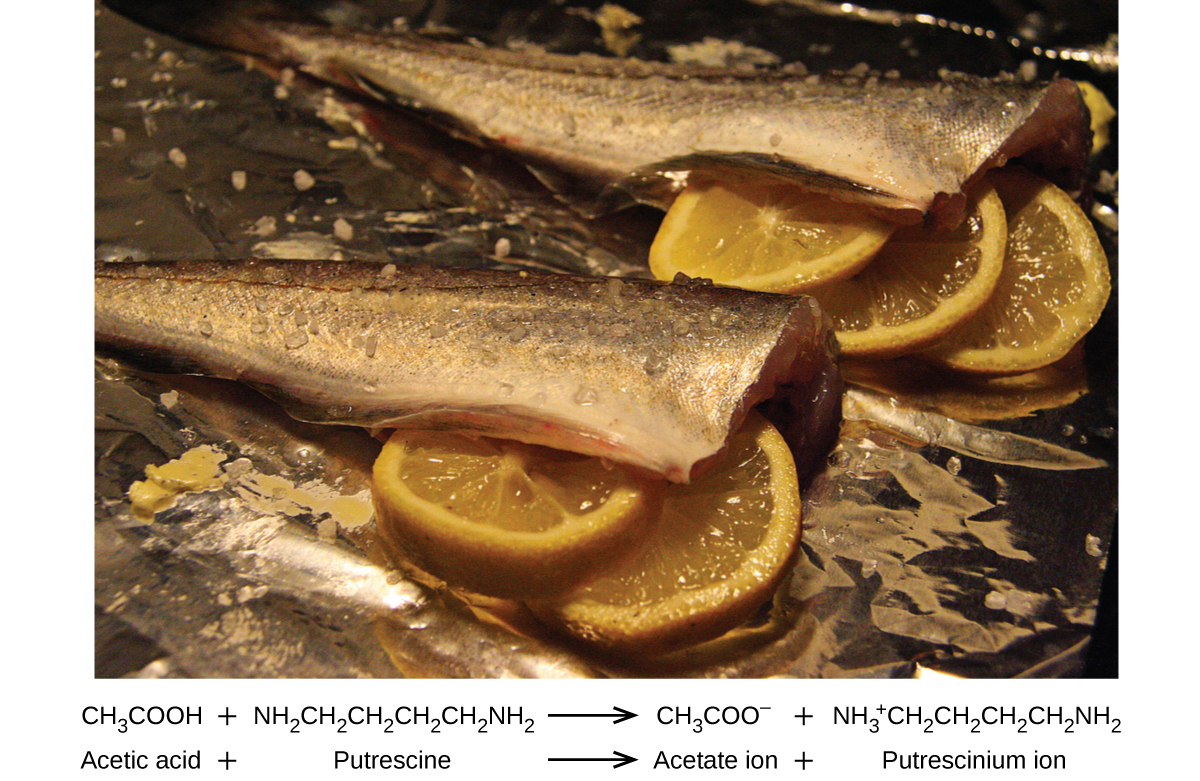
Many people like to put lemon juice or vinegar, both of which are acids, on cooked fish ((Figure)). It turns out that fish have volatile amines (bases) in their systems, which are neutralized by the acids to yield involatile ammonium salts. This reduces the odor of the fish, and also adds a “sour” taste that we seem to enjoy.
Pickling is a method used to preserve vegetables using a naturally produced acidic environment. The vegetable, such as a cucumber, is placed in a sealed jar submerged in a brine solution. The brine solution favors the growth of beneficial bacteria and suppresses the growth of harmful bacteria. The beneficial bacteria feed on starches in the cucumber and produce lactic acid as a waste product in a process called fermentation. The lactic acid eventually increases the acidity of the brine to a level that kills any harmful bacteria, which require a basic environment. Without the harmful bacteria consuming the cucumbers they are able to last much longer than if they were unprotected. A byproduct of the pickling process changes the flavor of the vegetables with the acid making them taste sour.
Explore the microscopic view of strong and weak acids and bases.
Oxidation-Reduction Reactions
Earth’s atmosphere contains about 20% molecular oxygen, O2, a chemically reactive gas that plays an essential role in the metabolism of aerobic organisms and in many environmental processes that shape the world. The term oxidation was originally used to describe chemical reactions involving O2, but its meaning has evolved to refer to a broad and important reaction class known as oxidation-reduction (redox) reactions. A few examples of such reactions will be used to develop a clear picture of this classification.
Some redox reactions involve the transfer of electrons between reactant species to yield ionic products, such as the reaction between sodium and chlorine to yield sodium chloride:
It is helpful to view the process with regard to each individual reactant, that is, to represent the fate of each reactant in the form of an equation called a half-reaction:
These equations show that Na atoms lose electrons while Cl atoms (in the Cl2 molecule) gain electrons, the “s” subscripts for the resulting ions signifying they are present in the form of a solid ionic compound. For redox reactions of this sort, the loss and gain of electrons define the complementary processes that occur:
In this reaction, then, sodium is oxidized and chlorine undergoes reduction. Viewed from a more active perspective, sodium functions as a reducing agent (reductant), since it provides electrons to (or reduces) chlorine. Likewise, chlorine functions as an oxidizing agent (oxidant), as it effectively removes electrons from (oxidizes) sodium.
Some redox processes, however, do not involve the transfer of electrons. Consider, for example, a reaction similar to the one yielding NaCl:
The product of this reaction is a covalent compound, so transfer of electrons in the explicit sense is not involved. To clarify the similarity of this reaction to the previous one and permit an unambiguous definition of redox reactions, a property called oxidation number has been defined.
Chemistry End of Chapter Exercises
1. Use the following equations to answer the next four questions:
i. ![]()
ii. ![]()
iii. ![]()
iv. ![]()
v. ![]()
(a) Which equation describes a physical change?
(b) Which equation identifies the reactants and products of a combustion reaction?
(c) Which equation is not balanced?
(d) Which is a net ionic equation?
2. Indicate what type, or types, of reaction each of the following represents:
(a) ![]()
(b) ![]()
(c) ![]()
(a) oxidation-reduction (addition); (b) acid-base (neutralization); (c) oxidation-reduction (combustion)
3. Indicate what type, or types, of reaction each of the following represents:
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
4. Silver can be separated from gold because silver dissolves in nitric acid while gold does not. Is the dissolution of silver in nitric acid an acid-base reaction or an oxidation-reduction reaction? Explain your answer.
It is an oxidation-reduction reaction because the oxidation state of the silver changes during the reaction.
5. Classify the following as acid-base reactions or oxidation-reduction reactions:
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
(e) ![]()
(f) ![]()
(a) acid-base; (b) oxidation-reduction: Na is oxidized, H+ is reduced; (c) oxidation-reduction: Mg is oxidized, Cl2 is reduced; (d) acid-base; (e) oxidation-reduction: P3− is oxidized, O2 is reduced; (f) acid-base
6. Identify the atoms that are oxidized and reduced, the change in oxidation state for each, and the oxidizing and reducing agents in each of the following equations:
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
(e) ![]()
(f) ![]()
7. Complete and balance the following acid-base equations:
(a) HCl gas reacts with solid Ca(OH)2(s).
(b) A solution of Sr(OH)2 is added to a solution of HNO3.
(a) ![]() (b)
(b) ![]()
8. Complete and balance the following acid-base equations:
(a) A solution of HClO4 is added to a solution of LiOH.
(b) Aqueous H2SO4 reacts with NaOH.
(c) Ba(OH)2 reacts with HF gas.
9. Complete and balance the equations for the following acid-base neutralization reactions. If water is used as a solvent, write the reactants and products as aqueous ions. In some cases, there may be more than one correct answer, depending on the amounts of reactants used.
(a) ![]()
(b) ![]()
(c) ![]()
(a) ![]() (b)
(b) ![]() (a solution of H2SO4); (c)
(a solution of H2SO4); (c) ![]()
10. When heated to 700–800 °C, diamonds, which are pure carbon, are oxidized by atmospheric oxygen. (They burn!) Write the balanced equation for this reaction.
11. The military has experimented with lasers that produce very intense light when fluorine combines explosively with hydrogen. What is the balanced equation for this reaction?
![]()
12. Write the molecular, total ionic, and net ionic equations for the following reactions:
(a) ![]()
(b) ![]()
13. Great Lakes Chemical Company produces bromine, Br2, from bromide salts such as NaBr, in Arkansas brine by treating the brine with chlorine gas. Write a balanced equation for the reaction of NaBr with Cl2.
![]()
14. In a common experiment in the general chemistry laboratory, magnesium metal is heated in air to produce MgO. MgO is a white solid, but in these experiments it often looks gray, due to small amounts of Mg3N2, a compound formed as some of the magnesium reacts with nitrogen. Write a balanced equation for each reaction.
15. Lithium hydroxide may be used to absorb carbon dioxide in enclosed environments, such as manned spacecraft and submarines. Write an equation for the reaction that involves 2 mol of LiOH per 1 mol of CO2. (Hint: Water is one of the products.)
![]()
16. Calcium propionate is sometimes added to bread to retard spoilage. This compound can be prepared by the reaction of calcium carbonate, CaCO3, with propionic acid, C2H5CO2H, which has properties similar to those of acetic acid. Write the balanced equation for the formation of calcium propionate.
17. Complete and balance the equations of the following reactions, each of which could be used to remove hydrogen sulfide from natural gas:
(a) ![]()
(b) ![]()
(a) ![]() (b)
(b) ![]()
18. Copper(II) sulfide is oxidized by molecular oxygen to produce gaseous sulfur trioxide and solid copper(II) oxide. The gaseous product then reacts with liquid water to produce liquid hydrogen sulfate as the only product. Write the two equations which represent these reactions.
19. Write balanced chemical equations for the reactions used to prepare each of the following compounds from the given starting material(s). In some cases, additional reactants may be required.
(a) solid ammonium nitrate from gaseous molecular nitrogen via a two-step process (first reduce the nitrogen to ammonia, then neutralize the ammonia with an appropriate acid)
(b) gaseous hydrogen bromide from liquid molecular bromine via a one-step redox reaction
(c) gaseous H2S from solid Zn and S via a two-step process (first a redox reaction between the starting materials, then reaction of the product with a strong acid)
(a) step 1: ![]() step 2:
step 2: ![]() (b)
(b) ![]() (c)
(c) ![]() and
and ![]()
20. Calcium cyclamate Ca(C6H11NHSO3)2 is an artificial sweetener used in many countries around the world but is banned in the United States. It can be purified industrially by converting it to the barium salt through reaction of the acid C6H11NHSO3H with barium carbonate, treatment with sulfuric acid (barium sulfate is very insoluble), and then neutralization with calcium hydroxide. Write the balanced equations for these reactions.
Footnotes
- 1The requirement of “charge balance” is just a specific type of “mass balance” in which the species in question are electrons. An equation must represent equal numbers of electrons on the reactant and product sides, and so both atoms and charges must be balanced.
Glossary
- acid
- substance that produces H3O+ when dissolved in water
- acid-base reaction
- reaction involving the transfer of a hydrogen ion between reactant species
- base
- substance that produces OH− when dissolved in water
- combustion reaction
- vigorous redox reaction producing significant amounts of energy in the form of heat and, sometimes, light
- half-reaction
- an equation that shows whether each reactant loses or gains electrons in a reaction.
- insoluble
- of relatively low solubility; dissolving only to a slight extent
- neutralization reaction
- reaction between an acid and a base to produce salt and water
- oxidation
- process in which an element’s oxidation number is increased by loss of electrons
- oxidation-reduction reaction
- (also, redox reaction) reaction involving a change in oxidation number for one or more reactant elements
- oxidation number
- (also, oxidation state) the charge each atom of an element would have in a compound if the compound were ionic
- oxidizing agent
- (also, oxidant) substance that brings about the oxidation of another substance, and in the process becomes reduced
- precipitate
- insoluble product that forms from reaction of soluble reactants
- precipitation reaction
- reaction that produces one or more insoluble products; when reactants are ionic compounds, sometimes called double-displacement or metathesis
- reduction
- process in which an element’s oxidation number is decreased by gain of electrons
- reducing agent
- (also, reductant) substance that brings about the reduction of another substance, and in the process becomes oxidized
- salt
- ionic compound that can be formed by the reaction of an acid with a base that contains a cation and an anion other than hydroxide or oxide
- single-displacement reaction
- (also, replacement) redox reaction involving the oxidation of an elemental substance by an ionic species
- soluble
- of relatively high solubility; dissolving to a relatively large extent
- solubility
- the extent to which a substance may be dissolved in water, or any solvent
- strong acid
- acid that reacts completely when dissolved in water to yield hydronium ions
- strong base
- base that reacts completely when dissolved in water to yield hydroxide ions
- weak acid
- acid that reacts only to a slight extent when dissolved in water to yield hydronium ions
- weak base
- base that reacts only to a slight extent when dissolved in water to yield hydroxide ions

