138 Uses of Radioisotopes
Learning Objectives
By the end of this section, you will be able to:
- List common applications of radioactive isotopes
Earlier in this chapter, we discussed some of the many uses of radioisotopes, including use in energy production; weapons production; and radiometric dating of the origin of various objects (such as archaeological artifacts or geological formations). There are many other uses of radioisotopes, as discussed below.
One useful feature of radioactive isotopes is that they have the same chemical properties as stable isotopes of the same element, but they emit radiation, which can be detected. Thus, if we replace one (or more) atom(s) with radioisotope(s) in a compound, we can track them by monitoring their radioactive emissions. This type of compound is called a radioactive tracer (or radioactive label). Radioisotopes are used to follow the paths of biochemical reactions or to determine how a substance is distributed within an organism. Radioactive tracers are used in applications as diverse as medical applications, measuring engine wear, analyzing the geological formation around oil wells, and much more.
Medical Use of Radioisotopes
Radioisotopes have revolutionized medical practice, where they are used extensively: see Table 138-1, for a list of many radioactive isotopes important to medicine. Radioisotopes are used both in the diagnosis and the treatment of disease, as discussed below.
| Table 138-1: Half-lives of Radioactive Isotopes Important to Medicine | |||
|---|---|---|---|
| Type1 | Decay Mode | Half-Life | Uses |
| F-18 | β+ decay | 110. minutes | PET scans |
| Co-60 | β decay, γ decay | 5.27 years | cancer treatment |
| Tc-99m | γ decay | 8.01 hours | scans of brain, lung, heart, bone |
| I-131 | β decay | 8.02 days | thyroid scans and treatment |
| Tl-201 | electron capture | 73 hours | heart and arteries scans; cardiac stress tests |
Radioisotopes in diagnosis of disease:
Four typical examples of radioactive tracers used in medicine are technetium-99 ![]() , thallium-201
, thallium-201 ![]() , iodine-131
, iodine-131 ![]() , and sodium-24
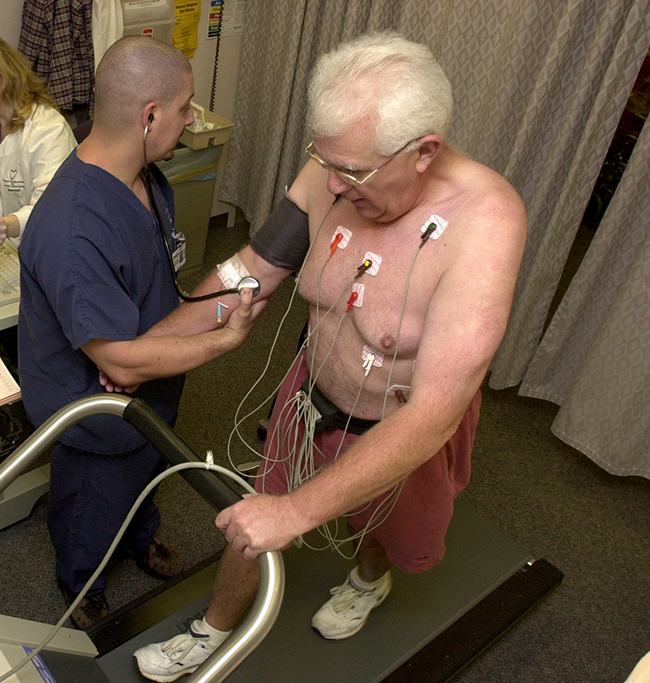
, and sodium-24 ![]() . Damaged tissues in the heart, liver, and lungs absorb certain compounds of technetium-99 preferentially. After it is injected, the location of the technetium compound, and hence the damaged tissue, can be determined by detecting the γ rays emitted by the Tc-99 isotope. Thallium-201 (Figure 138-1) becomes concentrated in healthy heart tissue, so the two isotopes, Tc-99 and Tl-201, are used together to study heart tissue. Iodine-131 concentrates in the thyroid gland, the liver, and some parts of the brain. It can therefore be used to monitor goiter and treat thyroid conditions, such as Grave’s disease, as well as liver and brain tumors. Salt solutions containing compounds of sodium-24 are injected into the bloodstream to help locate obstructions to the flow of blood.
. Damaged tissues in the heart, liver, and lungs absorb certain compounds of technetium-99 preferentially. After it is injected, the location of the technetium compound, and hence the damaged tissue, can be determined by detecting the γ rays emitted by the Tc-99 isotope. Thallium-201 (Figure 138-1) becomes concentrated in healthy heart tissue, so the two isotopes, Tc-99 and Tl-201, are used together to study heart tissue. Iodine-131 concentrates in the thyroid gland, the liver, and some parts of the brain. It can therefore be used to monitor goiter and treat thyroid conditions, such as Grave’s disease, as well as liver and brain tumors. Salt solutions containing compounds of sodium-24 are injected into the bloodstream to help locate obstructions to the flow of blood.
Radioisotopes used in medicine typically have short half-lives—for example, Tc-99m has a half-life of 6.01 hours. This makes Tc-99m essentially impossible to store and prohibitively expensive to transport, so it is made on-site instead. Hospitals and other medical facilities use Mo-99 (which is primarily extracted from U-235 fission products) to generate Tc-99m. Mo-99 undergoes β decay with a half-life of 66 hours, and the Tc-99m is then chemically extracted (Figure 138-2). The parent nuclide Mo-99 is part of a molybdate ion, ![]() when it decays, it forms the pertechnetate ion,
when it decays, it forms the pertechnetate ion, ![]() These two water-soluble ions are separated by column chromatography, with the higher charge molybdate ion adsorbing onto the alumina in the column, and the lower charge pertechnetate ion passing through the column in the solution. A few micrograms of Mo-99 can produce enough Tc-99m to perform as many as 10,000 tests.
These two water-soluble ions are separated by column chromatography, with the higher charge molybdate ion adsorbing onto the alumina in the column, and the lower charge pertechnetate ion passing through the column in the solution. A few micrograms of Mo-99 can produce enough Tc-99m to perform as many as 10,000 tests.
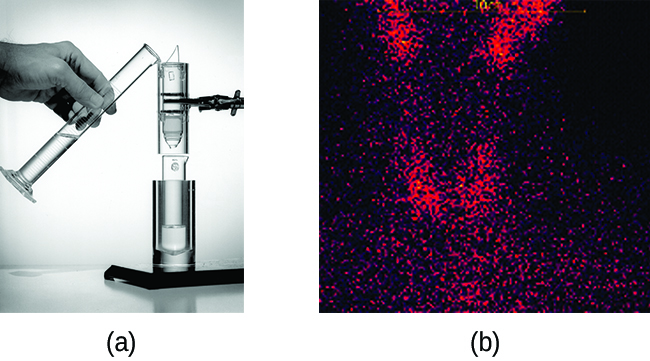
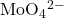 is retained by the matrix in the column, whereas the
is retained by the matrix in the column, whereas the 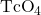 passes through and is collected. (b) Tc-99m was used in this scan of the neck of a patient with Grave’s disease. The scan shows the location of high concentrations of Tc-99m. (credit a: modification of work by the Department of Energy; credit b: modification of work by “MBq”/Wikimedia Commons)
passes through and is collected. (b) Tc-99m was used in this scan of the neck of a patient with Grave’s disease. The scan shows the location of high concentrations of Tc-99m. (credit a: modification of work by the Department of Energy; credit b: modification of work by “MBq”/Wikimedia Commons)Another extremely common use of medical isotopes in medical diagnoses is in “Positron Emission Topography” (PET) scans. PET scans most commonly involve the use of fluorine-18, which is a positron emitter with a half-life of about 110 minutes. Fluorine-18 is formed for medical use through the bombardment of oxygen-18 with high energy protons (in a cyclatron):
![]()
Fluorine-18 decomposes to release positrons (a form of antimatter). The positrons will immediately collide with one of the many electrons present; the matter and antimatter will cause mutual annihilation, releasing two ϒ particles of equal energy that travel in opposite directions:
![]()
This feature — that the two ϒ particles will travel in opposite directions but with equal energy — allows for the determination of their point of origin after detection. One reason why PET scans are so useful is that fluorine-18 can be incorporated into 2-fluorodeoxyglucose, an analogue of glucose, which is the fuel of choice for actively metabolizing tissues such as cancer cells and brain cells: these cells will accumulate [18F]-2-fluorodeoxyglucose, since it can be transported into the cells but they cannot metabolize it.
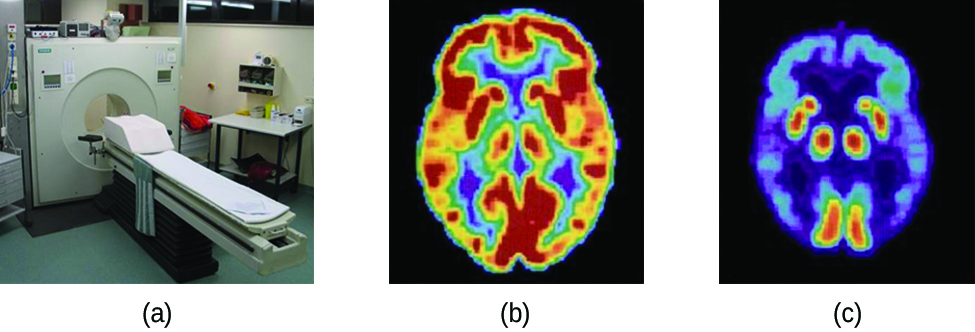
In a PET scan, different levels of gamma radiation produce different amounts of brightness and colors in the image, which can then be interpreted by a radiologist for diagnosis. PET scans can detect heart damage and heart disease, help diagnose Alzheimer’s disease, indicate the part of a brain that is affected by epilepsy, reveal cancer, show what stage it is, and how much it has spread, and whether treatments are effective (see Figure 135-3). The big advantage of PET scans is, unliked magnetic resonance imaging and X-rays which only show structural information, the PET scans reveal metabolic information. PET scans are now usually performed in conjunction with a computed tomography scan in order to overlay structural information with the metabolic information.
Radioisotopes in treatment of disease:
Radioisotopes can also be used, typically in higher doses than as a tracer, as treatment. Radiation therapy is the use of high-energy radiation to damage the DNA of cancer cells, which kills them or keeps them from dividing (Figure 138-4). A cancer patient may receive external beam radiation therapy delivered by a machine outside the body, or internal radiation therapy (brachytherapy) from a radioactive substance that has been introduced into the body. Note that chemotherapy is similar to internal radiation therapy in that the cancer treatment is injected into the body, but differs in that chemotherapy uses chemical rather than radioactive substances to kill the cancer cells.
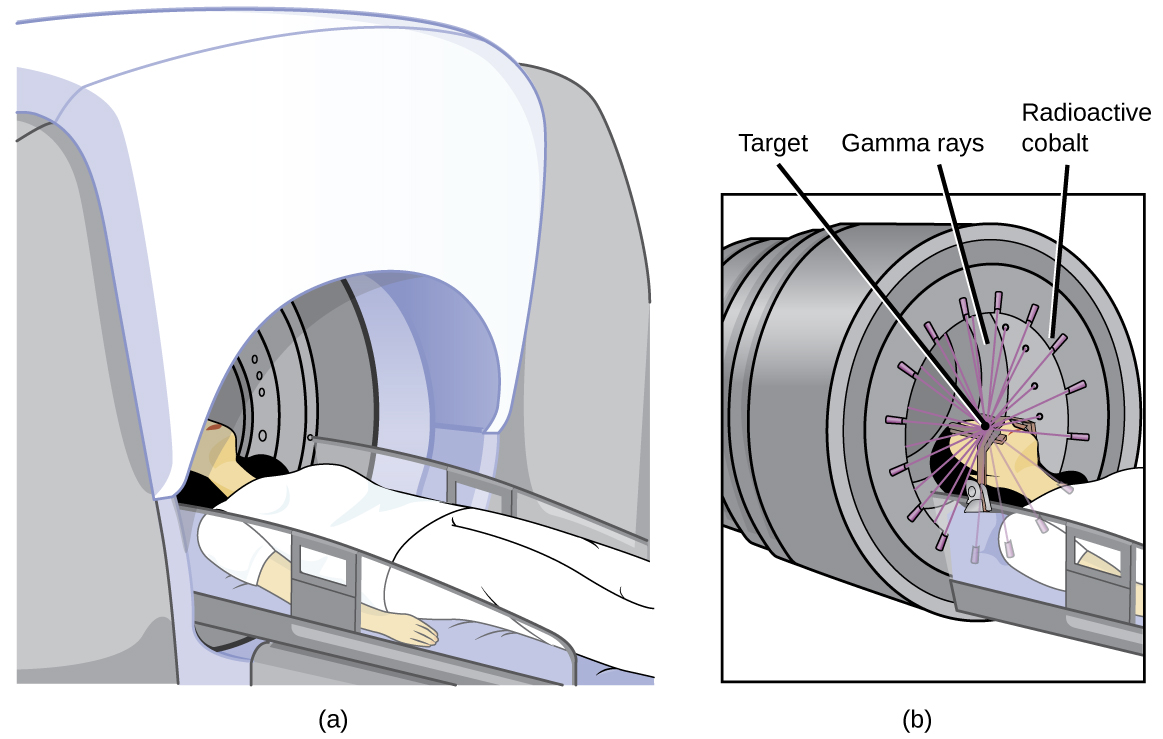
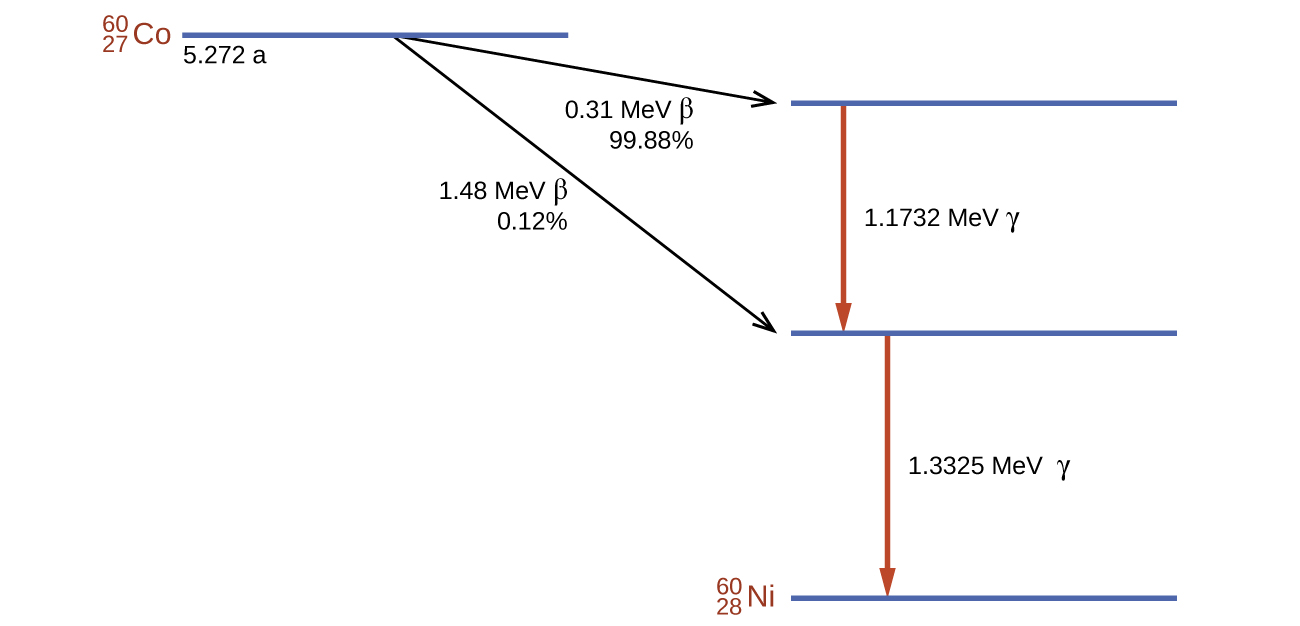
Cobalt-60 is a synthetic radioisotope produced by the neutron activation of Co-59, which then undergoes β decay to form Ni-60, along with the emission of γ radiation. The overall process is:
The overall decay scheme for this is shown graphically in Figure 138-5.
Commercial applications of radioactive materials:
Commercial applications of radioactive materials are equally diverse (Figure 138-6). They include determining the thickness of films and thin metal sheets by exploiting the penetration power of various types of radiation. Flaws in metals used for structural purposes can be detected using high-energy gamma rays from cobalt-60 in a fashion similar to the way X-rays are used to examine the human body. In one form of pest control, flies are controlled by sterilizing male flies with γ radiation so that females breeding with them do not produce offspring. Many foods are preserved by radiation that kills microorganisms that cause the foods to spoil.

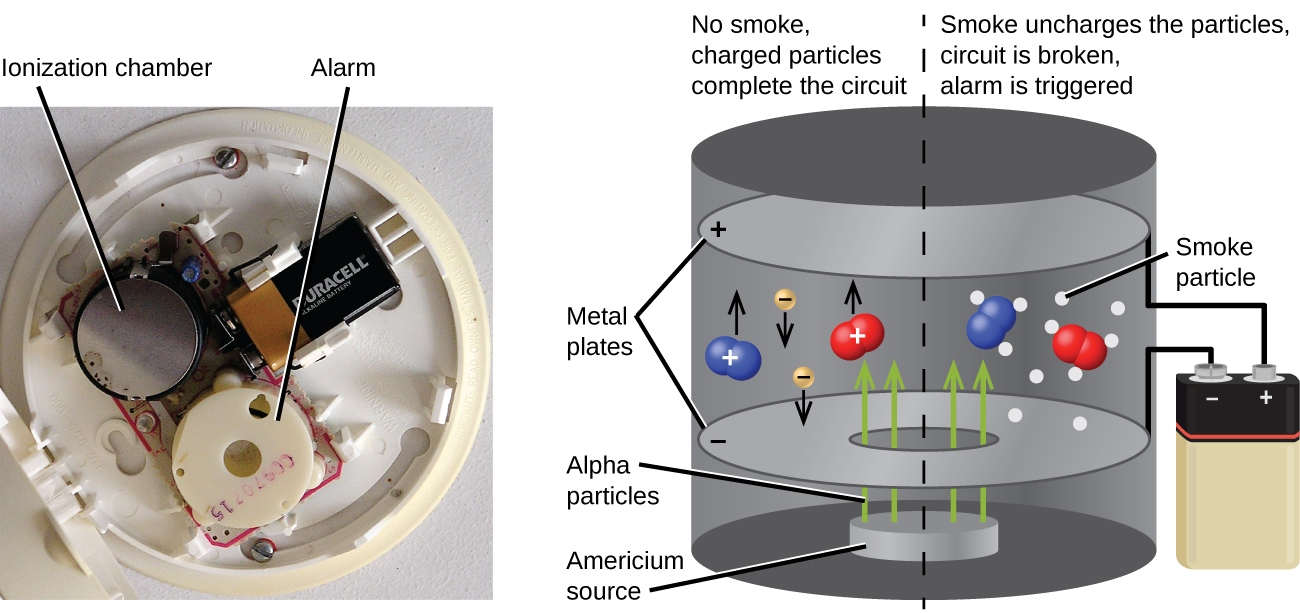
Americium-241, an α emitter with a half-life of 458 years, is used in tiny amounts in ionization-type smoke detectors (Figure 138-7). The α emissions from Am-241 ionize the air between two electrode plates in the ionizing chamber. A battery supplies a potential that causes movement of the ions, thus creating a small electric current. When smoke enters the chamber, the movement of the ions is impeded, reducing the conductivity of the air. This causes a marked drop in the current, triggering an alarm.
Use of radioisotopes in research:
Radioisotopes are used in diverse ways to study the mechanisms of chemical reactions in plants and animals. These include labeling fertilizers in studies of nutrient uptake by plants and crop growth, investigations of digestive and milk-producing processes in cows, and studies on the growth and metabolism of animals and plants.
For example, the radioisotope C-14 was used to elucidate the details of how photosynthesis occurs. The overall reaction is:
but the process is much more complex, proceeding through a series of steps in which various organic compounds are produced. In studies of the pathway of this reaction, plants were exposed to CO2 containing a high concentration of ![]() . At regular intervals, the plants were analyzed to determine which organic compounds contained carbon-14 and how much of each compound was present. From the time sequence in which the compounds appeared and the amount of each present at given time intervals, scientists learned more about the pathway of the reaction.
. At regular intervals, the plants were analyzed to determine which organic compounds contained carbon-14 and how much of each compound was present. From the time sequence in which the compounds appeared and the amount of each present at given time intervals, scientists learned more about the pathway of the reaction.
Key Concepts and Summary
Compounds known as radioactive tracers can be used to follow reactions, track the distribution of a substance, diagnose and treat medical conditions, and much more. Other radioactive substances are helpful for controlling pests, visualizing structures, providing fire warnings, and for many other applications. Hundreds of millions of nuclear medicine tests and procedures, using a wide variety of radioisotopes with relatively short half-lives, are performed every year in the US. Most of these radioisotopes have relatively short half-lives; some are short enough that the radioisotope must be made on-site at medical facilities. Radiation therapy uses high-energy radiation to kill cancer cells by damaging their DNA. The radiation used for this treatment may be delivered externally or internally.
Chemistry End of Unit 138 Exercises
Click here for the worked answers to the problems in this unit (and the rest of the Chapter).
- How can a radioactive nuclide be used to show that the equilibrium:
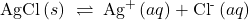
is a dynamic equilibrium?
Answer(s): Introduction of either radioactive Ag+ or radioactive Cl– into the solution containing the stated reaction, with subsequent time given for equilibration, will produce a radioactive precipitate that was originally devoid of radiation.
2. Technetium-99m has a half-life of 6.01 hours. If a patient injected with technetium-99m is safe to leave the hospital once 75% of the dose has decayed, when is the patient allowed to leave?
3. Iodine that enters the body is stored in the thyroid gland from which it is released to control growth and metabolism. The thyroid can be imaged if iodine-131 is injected into the body. In larger doses, I-133 is also used as a means of treating cancer of the thyroid. I-131 has a half-life of 8.70 days and decays by β− emission.
(a) Write an equation for the decay.
(b) How long will it take for 95.0% of a dose of I-131 to decay?
Answer(s): (a) ![]() (b) 37.6 days
(b) 37.6 days
Glossary
- chemotherapy
- similar to internal radiation therapy, but chemical rather than radioactive substances are introduced into the body to kill cancer cells
- external beam radiation therapy
- radiation delivered by a machine outside the body
- internal radiation therapy
- (also, brachytherapy) radiation from a radioactive substance introduced into the body to kill cancer cells
- radiation therapy
- use of high-energy radiation to damage the DNA of cancer cells, which kills them or keeps them from dividing
- radioactive tracer
- (also, radioactive label) radioisotope used to track or follow a substance by monitoring its radioactive emissions

