90 Review – Ionic Compounds in Aqueous Solution
Learning Objectives
By the end of this section, you will be able to:
- Recognize ionic species that are highly soluble in water.
- Identify the major ionic species present in aqueous solution of common salts, strong acids and strong bases.
-
Be able to identify strong acids and strong bases.
- Understand the terms “net ionic equation” and “spectator ions”.
Compounds in aqueous solution
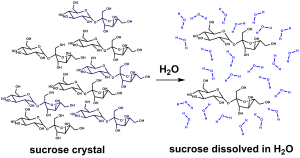
In this chapter we will mainly be dealing with aqueous solutions of acids, bases, and salts. It is important to recognize that compounds do not necessarily have the same structure in aqueous solution as they do in the solid state. When table sugar (sucrose) is dissolved in water, the sucrose (C12H22O12) does not change its chemical form: it is still present “intact” as C12H22O12.
C12H22O12(s) → C12H22O12(aq)
Note that all of the carbons, hydrogens, and oxygens are still bonded to the same atoms that they were bonded to in the solid state, the only difference is that now the molecules interact with water instead of with each other, as shown in Figure 90-1.
However, ionic compounds may not behave the same way. When potassium chloride (KCl, a common salt) is in solid form, each potassium ion forms ionic bonds with chloride ions, and vice versa. However, when potassium chloride is dissolved in water, most potassium and chloride ions are no longer directly associated with each other: instead, each ion is surrounded by several (4-6) water molecules (a “hydration shell”), as shown in Figure 90-2.
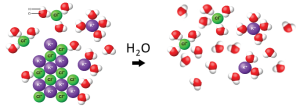
Thus, “KCl(aq)” does not mean that KCl is dissolved in water as such: in this case, it means that hydrated K+ and Cl– ions are present in aqueous solution. The concentration of undissociated KCl that is dissolved in water is, normally, essentially zero. (Note that some salts may not dissociate as completely as KCl in water).
Reactions between ionic compounds in aqueous solution:
Potassium hydroxide (KOH) and hydrochloric acid (HCl) will react in aqueous solution: the products of reaction are water and the neutral salt, potassium chloride (KCl). The reaction can be represented by a molecular equation as follows:
KOH(aq) + HCl(aq)→ KCl(aq) + H2O(l)
However, since we know that both the reactants (KOH and HCl) and the product (KCl) are ionic and dissociate completely into ions in aqueous solution, it is sometimes more informative to look at the complete ionic equation:
K+(aq) + OH–(aq) + H+(aq)+ Cl–(aq)→ K+(aq)+ Cl–(aq)+ H2O(l)
Upon inspection of the complete ionic equation, we can see that the K+ and Cl– ions did not undergo any change: they start as hydrated ions, and they are still hydrated ions when the reaction is over. In this reaction, the K+ and Cl– ions are known as spectator ions: they are required to maintain the charge neutrality of the solution, but they do not participate in the reaction (and, thus, do not change as the reaction proceeds). It is often more useful to represent this sort of reaction as a net ionic equation, which is not cluttered with the spectator ions:
OH–(aq) + H+(aq) → H2O(l)
Using the net ionic equation, it is easy to see that the reaction between aqueous KOH and HCl is really a neutralization reaction between the hydroxide and the hydrogen ion. For this reason, in this chapter we will usually find it more useful to focus on the net ionic equation, rather than the molecular equation or the complete ionic equation. (If you are not familiar with these different types of equations, you should consult Chapter 4, where they are introduced).
H3O+(aq) versus H+(aq):
You may be more familiar with the “hydronium ion”, written as H3O+(aq). The hydrogen ion, H+, is really just a nucleus (since the single electron of the hydrogen atom has been stripped away). (Note that these hydrogen ions are often referred to simply as protons, since that subatomic particle is the only component of cations derived from the most abundant hydrogen isotope, 1H.) Since there are no electrons shielding the proton in the nucleus, the hydrogen ion forms unusually strong noncovalent interactions with water. The relatively strong, transient associations of the H+ ion with several water molecules is known as its “hydration shell”. Chemists often write the hydrogen ion as H+(aq), not H3O+(aq), since it is understood that, when you write “(aq)”, the hydrogen ion is hydrated. In fact, the hydronium ion, H3O+, is itself hydrated (the H+ ion does not associate with only one water molecule). Thus two notations are used by chemists to represent that hydrogen ion in aqueous solution. The H3O+(aq) notation emphasizes the fact that the proton cannot exist on its own, and forms associations with water in aqueous solution. The H+(aq) means essentially the same thing but it makes it easier to balance reactions (since there is no “extra” water molecule in the formula). In this chapter we use the two conventions interchangeably, depending on what we are trying to emphasize.
In order to determine the net ionic equation for the reaction (if any) when two compounds are mixed in aqueous solution, it is important to know what form each compound takes once dissolved in water. This will allow you to determine the key reactive species versus the spectator ions. “Solubility rules” are useful to predict what ions will be present in the aqueous solutions of common compounds:
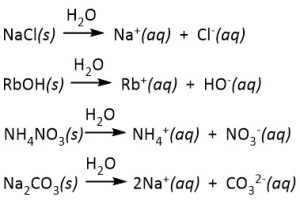
- all common salts of Group 1 elements and ammonium ion (NH4+) are extremely soluble in water. For example, the dissolution reactions of the common salts below can be written as indicated. The water (H2O) is written above the arrow since it is important for the dissolution reaction — it is the solvent, but it is not a stoichiometric reactant. The irreversible arrow (→) indicates that the dissolution reaction proceeds essentially to completion (i.e., no undissolved or undissociated salt will remain).
- all common nitrates (NO3–) are extremely soluble. For example,
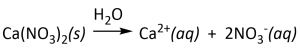
- most binary compounds of group 17 elements (other than fluorine, F) with metals are soluble. [Notable exceptions include those of silver, mercury(I)and lead]. For example:
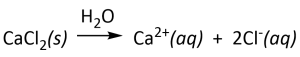
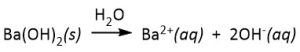
- strong bases will dissociate completely in solution to form hydroxide ion (OH–). Common strong bases (in aqueous solution) are the hydroxides of Group I and II metals except beryllium, and are listed in Table 90-1. For example, barium hydroxide would completely dissociate in water as indicated in the equation below:
| Table 90-1: Common strong bases in aqueous solution. | ||
|---|---|---|
| Base Name | Chemical Formula | Predominant Species in Water |
| Lithium hydroxide | LiOH | Li+(aq), OH–(aq) |
| Sodium hydroxide | NaOH | Na+(aq), OH–(aq) |
| Potassium hydroxide | KOH | K+(aq), OH–(aq) |
| Calcium hydroxide | Ca(OH)2 | Ca2+(aq), OH–(aq) |
| Strontium hydroxide | Sr(OH)2 | Sr2+(aq), OH–(aq) |
| Barium hydroxide | Ba(OH)2 | Ba2+(aq), OH–(aq) |
- strong acids will dissociate completely to donate their protons to water. For example, if hydrogen chloride (HCl) gas was bubbled into water, it would completely dissociate as indicated in the equation below:
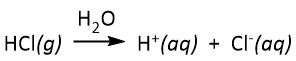
The most common strong acids (in aqueous solution) are listed Table 90-2.
| Table 90-2: Common strong acids in aqueous solution. | ||
|---|---|---|
| Acid Name | Chemical Formula | Predominant Species in Water |
| Hydrochloric acid | HCl | H+(aq), Cl–(aq) |
| Hydrobromic acid | HBr | H+(aq), Br–(aq) |
| Hydroiodic acid | HI | H+(aq), I–(aq) |
| perchloric acid | HClO4 | H+(aq), ClO4–(aq) |
| nitric acid | HNO3 | H+(aq), NO3–(aq) |
| sulfuric acid | H2SO4 | H+(aq), SO4–(aq) |
Ion Concentrations in Aqueous Solutions
Identify the major species that would be present in an 0.100 M aqueous solution of barium bromide (BaBr2). What are the concentrations of each species? (You can ignore water).
Solution:
Barium is a metal (Group 2) Bromine is a halogen (group 17), and so one would expect the compound to be highly soluble in water: only Ba2+(aq) and Br–(aq) would be present. There would be negligible undissolved BaBr2 in solution.
Given the stoichiometry of the ions present in the compound, 0.100 M of barium bromide dissolved in water would produce a solution with 0.100 M Ba2+and 0.200 M Br–.
Check your learning:
(a) Identify the major species that would be present in an 0.010 M aqueous solution of calcium hydroxide Ca(OH)2. What are the concentrations of each species? (You can ignore water).
(b) Identify the major species that would be present in an 0.020 M aqueous solution of beryllium nitrate, Be(NO3)2. What are the concentrations of each species? (You can ignore water).
Solution:
(a) The aqueous solution would contain 0.010 M Ca2+(aq) and 0.020 M OH–(aq). [Note that there would be negligible undissociated Ca(OH)2 present in solution.]
(b) The aqueous solution would contain 0.020 M Be2+(aq) and 0.040 M NO3–(aq). [Note that there would be negligible undissociated Be(NO3)2 present in solution.]
Key Concepts and Summary
Chemical reactions in aqueous solution are conveniently represented using net ionic equations, which clearly indicate the reacting species and ignore any spectator ions. Using the rules of solubility, it is possible to predict which ions are present in aqueous solution, which is a first step towards determining the net ionic equation.
Section 90 Practice Problems
Click on this link to view the answers to these problems (as a downloadable pdf).
1. Identify the major species that would be present in aqueous solutions of each the following compounds.
(a) HI
(b) CsCl
(c) NH4NO3
(d) Sr(OH)2
Answer(s):
(a) H2O(l), H+(aq), and I–(aq)
(b) H2O(l), Cs+(aq), and Cl–(aq)
(c) H2O(l), NH4+(aq), and NO3–(aq)
(d) H2O(l), Sr2+(aq), and OH–(aq)
2. Determine the concentrations of all major species in the aqueous solutions indicated below. (You can ignore the solvent, water, which would be present at a relatively constant 55.5 M).
(a) 0.100 M HI
(b) 0.050 M CsCl
(c) 0.020 M NH4NO3
(d) 0.200 M Sr(OH)2
Answer(s):
(a) 0.100 M H+(aq) and 0.100 M I–(aq)
(b) 0.050 M Cs+(aq) and 0.050 M Cl–(aq)
(c) 0.020 M NH4+(aq) and 0.020 M NO3–(aq)
(d) 0.200 M Sr2+(aq) and 0.400 M OH–(aq)

