72 The Second Law of Thermodynamics
Learning Objectives
By the end of this section, you will be able to:
- State and explain the second law of thermodynamics
- Calculate entropy changes for phase transitions under standard conditions
In the quest to identify a property that may reliably predict the spontaneity of a process, a promising candidate has been identified: entropy. Processes that involve an increase in entropy of the system (ΔS > 0) are very often spontaneous; however, examples to the contrary are plentiful. By expanding consideration of entropy changes to include the surroundings, we may reach a significant conclusion regarding the relation between this property and spontaneity. In thermodynamic models, the system and surroundings comprise everything, that is, the universe, and so the following is true:
To illustrate this relation, consider again the process of heat flow between two objects, one identified as the system and the other as the surroundings. There are three possibilities for such a process:
- The objects are at different temperatures, and heat flows from the hotter to the cooler object. This is always observed to occur spontaneously. Designating the hotter object as the system and invoking the definition of entropy yields the following:
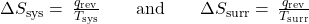
The magnitudes of −qrev and qrev are equal, their opposite arithmetic signs denoting loss of heat by the system and gain of heat by the surroundings. Since Tsys > Tsurr in this scenario, the entropy decrease of the system will be less than the entropy increase of the surroundings, and so the entropy of the universe will increase:
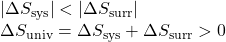
- The objects are at different temperatures, and heat flows from the cooler to the hotter object. This is never observed to occur spontaneously. Again designating the hotter object as the system and invoking the definition of entropy yields the following:
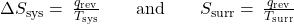
The arithmetic signs of qrev denote the gain of heat by the system and the loss of heat by the surroundings. The magnitude of the entropy change for the surroundings will again be greater than that for the system, but in this case, the signs of the heat changes (that is, the direction of the heat flow) will yield a negative value for ΔSuniv. This process involves a decrease in the entropy of the universe.
- The objects are at essentially the same temperature, Tsys ≈ Tsurr, and so the magnitudes of the entropy changes are essentially the same for both the system and the surroundings. In this case, the entropy change of the universe is zero, and the system is at equilibrium.
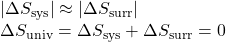
These results lead to a profound statement regarding the relation between entropy and spontaneity known as the second law of thermodynamics: all spontaneous changes cause an increase in the entropy of the universe. A summary of these three relations is provided in Table 72-1.
| Table 72-1: The Second Law of Thermodynamics | |
|---|---|
| ΔSuniv > 0 | spontaneous |
| ΔSuniv < 0 | nonspontaneous (spontaneous in opposite direction) |
| ΔSuniv = 0 | at equilibrium |
For many realistic applications, the surroundings are vast in comparison to the system. In such cases, the heat gained or lost by the surroundings as a result of some process represents a very small, nearly infinitesimal, fraction of its total thermal energy. For example, combustion of a fuel in air involves transfer of heat from a system (the fuel and oxygen molecules undergoing reaction) to surroundings that are infinitely more massive (the earth’s atmosphere). As a result, qsurr is a good approximation of qrev, and the second law may be stated as the following:
We may use this equation to predict the spontaneity of a process as illustrated in the Example below.
EXAMPLE:
Will Ice Spontaneously Melt?
The entropy change for the process
is 22.1 J K-1 and requires that the surroundings transfer 6.00 kJ of heat to the system. Is the process spontaneous at −10.00 °C? Is it spontaneous at +10.00 °C?
Solution
We can assess the spontaneity of the process by calculating the entropy change of the universe. If ΔSuniv is positive, then the process is spontaneous. At both temperatures, ΔSsys = 22.1 J K-1 and qsurr = −6.00 kJ.
At −10.00 °C (263.15 K), the following is true:
![Rendered by QuickLaTeX.com \[ \begin{array}{cc} \Delta S_{\text{univ}} & = \Delta S_{\text{sys}} + \Delta S_{\text{surr}} = \Delta S_{\text{sys}} + \frac{q_{\text{surr}}}{T} \\ \\ & = 22.1 \, \text{J/K} + \frac{-6.00 \times 10^{3} \, \text{J}}{263.15 \, \text{K}} \\ \\ & = 22.1 \, \text{J/K} + \frac{-6000 \, \text{J}}{263.15 \, \text{K}} \\ \\ & = 22.1 \, \text{J/K} - 22.8 \, \text{J/K} \\ \\ & = -0.7 \, \text{J/K} \end{array} \]](https://pressbooks.openedmb.ca/app/uploads/quicklatex/quicklatex.com-65f5abeecddad009398fad18f8de8875_l3.png)
ΔSuniv < 0, so melting is nonspontaneous (not spontaneous) at −10.0 °C.
At 10.00 °C (283.15 K), the following is true:
![Rendered by QuickLaTeX.com \[ \begin{array}{cc} \Delta S_{\text{univ}} & = \Delta S_{\text{sys}} + \Delta S_{\text{surr}} = \Delta S_{\text{sys}} + \frac{q_{\text{surr}}}{T} \\ \\ & = 22.1 \, \text{J/K} + \frac{-6.00 \times 10^{3} \, \text{J}}{283.15 \, \text{K}} \\ \\ & = 22.1 \, \text{J/K} + \frac{-6000 \, \text{J}}{283.15 \, \text{K}} \\ \\ & = 22.1 \, \text{J/K} - 21.2 \, \text{J/K} \\ \\ & = 0.9 \, \text{J/K} \end{array} \]](https://pressbooks.openedmb.ca/app/uploads/quicklatex/quicklatex.com-ea9760da8fac46a39f2bf0d1abff58eb_l3.png)
Check Your Learning
Using this information, determine if liquid water will spontaneously freeze at the same temperatures. What can you say about the values of ΔSuniv?
Key Concepts and Summary
- The second law of thermodynamics states that a spontaneous process increases the entropy of the universe, ΔSuniv > 0. If ΔSuniv < 0, the process is nonspontaneous, and if ΔSuniv = 0, the system is at equilibrium.
Key Equations
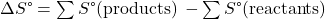
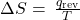
- ΔSuniv = ΔSsys + ΔSsurr
Section 72 Practice Problems
72-1. Hydrogen iodide (HI) has a normal boiling point of –35.4 °C, and its ΔHvap is 21.16 kJ mol-1. Calculate the molar entropy of vaporization (ΔSvap).
72-2. What is the total entropy change (i.e., system plus surroundings) when 17.9 g of water (∆Hfus =6.01 kJ mol-1) freezes at 0.0 °C in a freezer compartment whose temperature is held at -29.0 °C? Is this process spontaneous?
72-3. What is the total entropy change (i.e. system plus surroundings) when 68.2 g of ice (∆Hfus = 6.01 kJ mol-1) melts at 0.0 oC on a counter in the kitchen whose temperature is 20.0 oC? Is this process spontaneous?
72-4. What is the total entropy change when 1.76 mol of water (∆Hfus = 6.01 kJ mol-1) melts at 0.0 oC outside in Winnipeg when it is -35.0 oC outside? Is this process spontaneous?
Glossary
- second law of thermodynamics
- all spontaneous processes involve an increase in the entropy of the universe

