94 Relative strengths of acids and bases: effect of molecular structure
Learning Objectives
By the end of this section, you will be able to:
- Rationalize trends in acid–base strength in relation to molecular structure
Examples of common acids and bases are listed in Table 94-1. It is important to be able to recognize acids and bases on the basis of their structures. In this chapter we will consider the effect of molecular structure on the acidity or basicity of a compound.
| Table 94-1: Common acids and bases | |
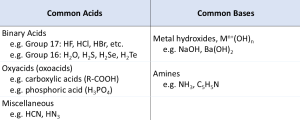 |
Relative Strengths of Binary Acids and Bases
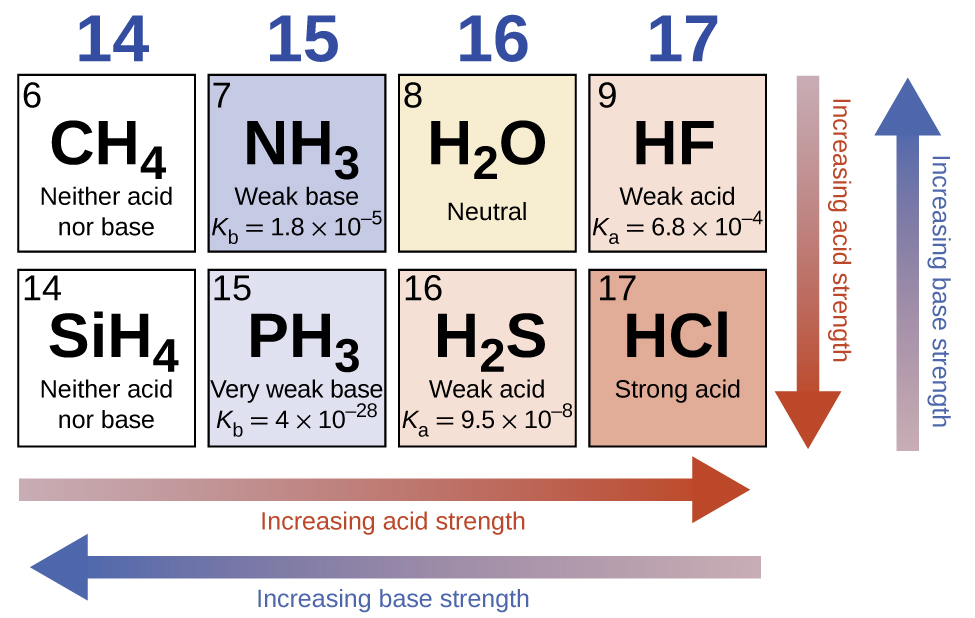
A “binary acid” is one that has only two types of atoms: hydrogen and another element. In the absence of any leveling effect, the acid strength of binary compounds of hydrogen with nonmetals (A) increases as the H-A bond strength decreases down a group in the periodic table. For group 17, the order of increasing acidity is HF < HCl < HBr < HI. Likewise, for group 16, the order of increasing acid strength is H2O < H2S < H2Se < H2Te.
Across a row in the periodic table, the acid strength of binary hydrogen compounds increases with increasing electronegativity of the nonmetal atom because the stability of the product increases. Thus, the order of increasing acidity (for removal of one proton) across the second row is CH4 < NH3 < H2O < HF; across the third row, it is SiH4 < PH3 < H2S < HCl (see Figure 94-1).
Ternary Acids and Bases
Ternary compounds composed of hydrogen, oxygen, and some third element (“E”) may be structured as depicted in the image below. In these compounds, the central E atom is bonded to one or more O atoms, and at least one of the O atoms is also bonded to an H atom, corresponding to the general molecular formula OmE(OH)n. These compounds may be acidic, basic, or amphoteric depending on the properties of the central E atom. Examples of such compounds include sulfuric acid, O2S(OH)2, sulfurous acid, OS(OH)2, nitric acid, O2NOH, perchloric acid, O3ClOH, aluminum hydroxide, Al(OH)3, calcium hydroxide, Ca(OH)2, and potassium hydroxide, KOH:
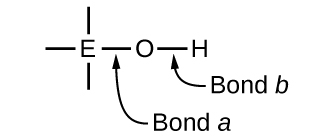
If the central atom, E, has a low electronegativity, its attraction for electrons is low. Little tendency exists for the central atom to form a strong covalent bond with the oxygen atom, and bond a between the element and oxygen is more readily broken than bond b between oxygen and hydrogen. Hence bond a is ionic, hydroxide ions are released to the solution, and the material behaves as a base—this is the case with Ca(OH)2 and KOH. Lower electronegativity is characteristic of the more metallic elements; hence, the metallic elements form ionic hydroxides that are by definition basic compounds.
If, on the other hand, the atom E has a relatively high electronegativity, it strongly attracts the electrons it shares with the oxygen atom, making bond a relatively strongly covalent. The oxygen-hydrogen bond, bond b, is thereby weakened because electrons are displaced toward E. Bond b is polar and readily releases hydrogen ions to the solution, so the material behaves as an acid. High electronegativities are characteristic of the more nonmetallic elements. Thus, nonmetallic elements form covalent compounds containing acidic −OH groups that are called oxyacids.
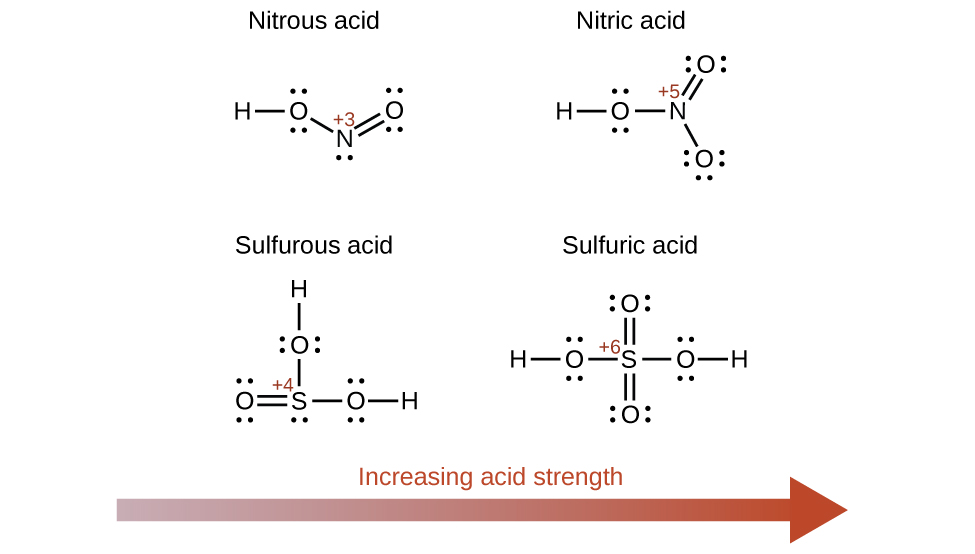
Increasing the oxidation number of the central atom E also increases the acidity of an oxyacid because this increases the attraction of E for the electrons it shares with oxygen and thereby weakens the O-H bond. Sulfuric acid, H2SO4, or O2S(OH)2 (with a sulfur oxidation number of +6), is more acidic than sulfurous acid, H2SO3, or OS(OH)2 (with a sulfur oxidation number of +4). Likewise nitric acid, HNO3, or O2NOH (N oxidation number = +5), is more acidic than nitrous acid, HNO2, or ONOH (N oxidation number = +3). In each of these pairs, the oxidation number of the central atom is larger for the stronger acid (Figure 94-2).
Hydroxy compounds of elements with intermediate electronegativities and relatively high oxidation numbers (for example, elements near the diagonal line separating the metals from the nonmetals in the periodic table) are usually amphoteric. This means that the hydroxy compounds act as acids when they react with strong bases and as bases when they react with strong acids. The amphoterism of aluminum hydroxide, which commonly exists as the hydrate Al(H2O)3(OH)3, is reflected in its solubility in both strong acids and strong bases. In strong bases, the relatively insoluble hydrated aluminum hydroxide, Al(H2O)3(OH)3, is converted into the soluble ion, ![]() by reaction with hydroxide ion:
by reaction with hydroxide ion:
In this reaction, a proton is transferred from one of the aluminum-bound H2O molecules to a hydroxide ion in solution. The Al(H2O)3(OH)3 compound thus acts as an acid under these conditions. On the other hand, when dissolved in strong acids, it is converted to the soluble ion ![]() by reaction with hydronium ion:
by reaction with hydronium ion:
In this case, protons are transferred from hydronium ions in solution to Al(H2O)3(OH)3, and the compound functions as a base.
Key Equations
Chemistry End of Unit 94 Exercises
1. Use this list of important industrial compounds (and Figure 93-2) to answer the following questions regarding: CaO, Ca(OH)2, CH3CO2H, CO2, HCl, H2CO3, HF, HNO2, HNO3, H3PO4, H2SO4, NH3, NaOH, Na2CO3.
(a) Identify the strong Brønsted-Lowry acids and strong Brønsted-Lowry bases.
(b) List those compounds in (a) that can behave as Brønsted-Lowry acids with strengths lying between those of H3O+ and H2O.
(c) List those compounds in (a) that can behave as Brønsted-Lowry bases with strengths lying between those of H2O and OH−.
2. Explain why the ionization constant, Ka, for H2SO4 is larger than the ionization constant for H2SO3.
Answer(s): The oxidation state of the sulfur in H2SO4 is greater than the oxidation state of the sulfur in H2SO3.
3. Explain why HI is a stronger acid than HF.
4. Predict which acid in each of the following pairs is the stronger and explain your reasoning for each.
(a) H2O or HF
(b) B(OH)3 or Al(OH)3
(c) ![]() or
or ![]()
(d) NH3 or H2S
(e) H2O or H2Te
5. Predict which compound in each of the following pairs of compounds is more acidic and explain your reasoning for each.
(a) HSO4– or HSeO4–
(b) NH3 or H2O
(c) PH3 or HI
(d) NH3 or PH3
(e) H2S or HBr
Answer(s): (a) ![]() higher electronegativity of the central ion. (b) H2O; oxygen is more electronegative than nitrogen. In addition, we know that NH3 is a base and water is neutral, or we can compare Ka values. (c) HI; PH3 is weaker than HCl (acidity increases as you move across a row); HCl is weaker than HI (acid strength increases as you move down a period). Thus, PH3 is weaker than HI. (d) PH3; in binary compounds of hydrogen with nonmetals, the acidity increases for the element lower in a group. (e) HBr; in a period, the acidity increases from left to right; in a group, it increases from top to bottom. Br is to the right and below S, so HBr is the stronger acid.
higher electronegativity of the central ion. (b) H2O; oxygen is more electronegative than nitrogen. In addition, we know that NH3 is a base and water is neutral, or we can compare Ka values. (c) HI; PH3 is weaker than HCl (acidity increases as you move across a row); HCl is weaker than HI (acid strength increases as you move down a period). Thus, PH3 is weaker than HI. (d) PH3; in binary compounds of hydrogen with nonmetals, the acidity increases for the element lower in a group. (e) HBr; in a period, the acidity increases from left to right; in a group, it increases from top to bottom. Br is to the right and below S, so HBr is the stronger acid.
6. Rank the compounds in each of the following groups in order of increasing acidity or basicity, as indicated, and explain the order you assign.
(a) acidity: HCl, HBr, HI
(b) basicity: H2O, OH−, H−, Cl−
(c) basicity: Mg(OH)2, Si(OH)4, ClO3(OH) (Hint: Formula could also be written as HClO4.)
(d) acidity: HF, H2O, NH3, CH4
7. Rank the compounds in each of the following groups in order of increasing acidity or basicity, as indicated, and explain the order you assign.
(a) acidity: NaHSO3, NaHSeO3, NaHSO4
(b) basicity: ![]()
![]()
![]()
(c) acidity: HOCl, HOBr, HOI
(d) acidity: HOCl, HOClO, HOClO2, HOClO3
(e) basicity: ![]() HS−, HTe−,
HS−, HTe−, ![]()
(f) basicity: BrO−, ![]()
![]()
![]()
Answer(s): (a) NaHSeO3 < NaHSO3 < NaHSO4; in polyoxy acids, the more electronegative central element—S, in this case—forms the stronger acid. The larger number of oxygen atoms on the central atom (giving it a higher oxidation state) also creates a greater release of hydrogen atoms, resulting in a stronger acid. As a salt, the acidity increases in the same manner. (b) ![]() the basicity of the anions in a series of acids will be the opposite of the acidity in their oxyacids. The acidity increases as the electronegativity of the central atom increases. Cl is more electronegative than Br, and I is the least electronegative of the three. (c) HOI < HOBr < HOCl; in a series of the same form of oxyacids, the acidity increases as the electronegativity of the central atom increases. Cl is more electronegative than Br, and I is the least electronegative of the three. (d) HOCl < HOClO < HOClO2 < HOClO3; in a series of oxyacids of the same central element, the acidity increases as the number of oxygen atoms increases (or as the oxidation state of the central atom increases). (e)
the basicity of the anions in a series of acids will be the opposite of the acidity in their oxyacids. The acidity increases as the electronegativity of the central atom increases. Cl is more electronegative than Br, and I is the least electronegative of the three. (c) HOI < HOBr < HOCl; in a series of the same form of oxyacids, the acidity increases as the electronegativity of the central atom increases. Cl is more electronegative than Br, and I is the least electronegative of the three. (d) HOCl < HOClO < HOClO2 < HOClO3; in a series of oxyacids of the same central element, the acidity increases as the number of oxygen atoms increases (or as the oxidation state of the central atom increases). (e) ![]()
![]() and
and ![]() are anions of weak bases, so they act as strong bases toward H+.
are anions of weak bases, so they act as strong bases toward H+. ![]() and HS− are anions of weak acids, so they have less basic character. In a periodic group, the more electronegative element has the more basic anion. (f)
and HS− are anions of weak acids, so they have less basic character. In a periodic group, the more electronegative element has the more basic anion. (f) ![]() with a larger number of oxygen atoms (that is, as the oxidation state of the central ion increases), the corresponding acid becomes more acidic and the anion consequently less basic.
with a larger number of oxygen atoms (that is, as the oxidation state of the central ion increases), the corresponding acid becomes more acidic and the anion consequently less basic.
Glossary
- oxyacid
- ternary compound with acidic properties, molecules of which contain a central nonmetallic atom bonded to one or more O atoms, at least one of which is bonded to an ionizable H atom

