95 Hydrolysis of Salts
Learning Objectives
By the end of this section, you will be able to:
- Write the reactions between an acid (weak or strong) and a base (weak or strong), to form water and a salt.
- Predict whether a salt solution will be acidic, basic, or neutral
- Calculate the concentrations of the various species in a salt solution
- Describe the acid ionization of hydrated metal ions
Neutralization Reactions
The reaction between an acid and a base is called an acid-base neutralization reaction. For example, if equivalent quantities of sodium hydroxide (NaOH, a strong base) and hydrogen chloride (HCl, a strong acid) react, the acid and the base are neutralized. The molecular equation for the reaction is as follows:
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Note that the major products of the neutralization reaction between an acid and a base are water and a salt (an ionic compound composed of cations and anions).
Aqueous salt solutions may be acidic, basic, or neutral, depending on the relative acid-base strengths of the salt’s constituent ions. In the case when equimolar quantities of a strong acid and a strong base react, the resulting solution is neutral (i.e., sodium chloride is a “neutral salt”). The complete ionic equation for the reaction between a strong acid (fully dissociated to form protons) and a strong base (fully dissociated to form hydroxide anions) is as follows:
Na+(aq) + OH–(aq) + H+(aq) + Cl–(aq) → H2O(l) + Na+(aq) + + Cl–(aq)
Since neither the sodium ions (Na+) nor the chloride ions (Cl–) will react further with water, the solution is neutral and sodium chloride (NaCl) is said to be a “neutral salt”.
However, some salts are capable of undergoing further reaction with water to form acidic or basic aqueous solutions: the cations, anions, or both can undergo acid-base reactions with water. When ions interact with water to form acidic or basic solutions, they are said to “hydrolyze” in water, or to undergo “hydrolysis”.
If the resulting solution is acidic, the salt is said to be an “acidic salt”. If the resulting solution is basic, the salt is said to be a “basic salt”. The ultimate pH of the solution can be determined based on the Ka and/or Kb values of the cations and anions in the salt.
Salts with Acidic Ions
A classic example of an acidic salt is ammonium chloride. Ammonium chloride is a salt that could be formed by the reaction between the weak base ammonia (NH3) and the strong acid hydrochloric acid (HCl):
NH3(aq) + HCl(aq) → NH4Cl(aq)
Recall that ammonium chloride is fully dissociated in water: ammonium ions (NH4+) and chloride ions (Cl–) are the major species. The ammonium ion (NH4+) is the conjugate acid of the weak base ammonia (NH3) and is, itself, a weak acid. (Recall that the conjugate acids of weak bases are weak acids.) Thus the ammonium ion will, to a small extent, dissociate to transfer protons to water: it is said to undergo acid ionization (or acid hydrolysis):
The chloride ion is the conjugate base of hydrochloric acid, and so its base ionization (or base hydrolysis) reaction is represented by
Since HCl is a strong acid, Ka is immeasurably large. Thus, chloride is an extremely weak base, with a Kb ≈ 0. Chloride ions do not undergo significant reaction with water and do not affect the pH of the aqueous solution. (The chloride ion is “pH neutral”).
Thus, dissolving ammonium chloride in water yields a solution of weak acid cations (NH4+) and inert anions (Cl−), resulting in an acidic solution. Ammonium chloride is said to be an “acidic salt”. The pH of a known concentration of ammonium chloride can be determined, since the Ka of the ammonium ion can be determined from the Kb of ammonia (NH3). As discussed previously,
Ka x Kb = Kw
Calculating the pH of an Acidic Salt Solution
Aniline is an amine that is used to manufacture dyes. It is isolated as anilinium chloride, [C6H5NH3+]Cl– a salt prepared by the reaction of the weak base aniline and hydrochloric acid. The anilinium ion is a weak acid (analogous to ammonium, NH4+). What is the pH of a 0.233 M solution of anilinium chloride?
Solution
The Ka for anilinium ion is derived from the Kb for its conjugate base, aniline (see Appendix H):
Using the provided information, an ICE table for this system is prepared:
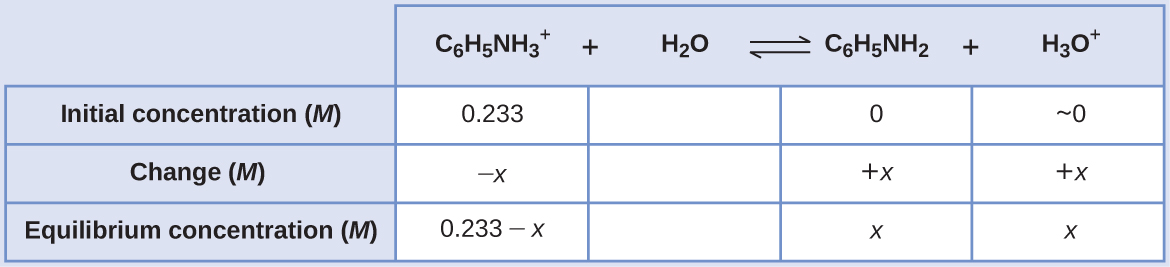
Substituting these equilibrium concentration terms into the Ka expression gives
Assuming x << 0.233, the equation is simplified and solved for x:
The ICE table defines x as the hydronium ion molarity, and so the pH is computed as
Check Your Learning
What is the hydronium ion concentration in a 0.100 M solution of ammonium nitrate, NH4NO3, a salt composed of the ions ![]() and
and ![]() Which is the stronger acid
Which is the stronger acid ![]() or
or ![]()
Salts with Basic Ions
As another example, consider dissolving sodium acetate in water:
The sodium ion does not undergo appreciable acid or base ionization and has no effect on the solution pH. This may seem obvious from the ion’s formula, which indicates no hydrogen or oxygen atoms, but some dissolved metal ions function as weak acids, as addressed later in this section.
The acetate ion, CH3CO2– is the conjugate base of acetic acid, CH3CO2H, and so its base ionization (or base hydrolysis) reaction is represented by
Because acetic acid is a weak acid, its Ka is measurable and acetate ion is a weak base (Kb > 0).
Dissolving sodium acetate in water yields a solution of inert cations (Na+) and weak base anions (CH3CO2–) resulting in a basic solution.
Equilibrium in a Solution of a Salt of a Weak Acid and a Strong Base
Determine the acetic acid (CH3CO2H) concentration in a solution with [CH3CO2–] = 0.050 M and [OH−] = 2.5 x 10−6M at equilibrium. The reaction is:
Solution
The provided equilibrium concentrations and a value for the equilibrium constant will permit calculation of the missing equilibrium concentration. The process in question is the base ionization of acetate ion, for which
Substituting the available values into the Kb expression gives
Solving the above equation for the acetic acid molarity yields [CH3CO2H] = 1.1 x 10−5M.
Check Your Learning
What is the pH of a 0.083 M solution of NaCN?
Salts with Acidic and Basic Ions
Some salts are composed of both acidic and basic ions, and so the pH of their solutions will depend on the relative strengths of these two species. Likewise, some salts contain a single ion that is amphiprotic, and so the relative strengths of this ion’s acid and base character will determine its effect on solution pH. For both types of salts, a comparison of the Ka and Kb values allows prediction of the solution’s acid-base status, as illustrated in the following example exercise.
Determining the Acidic or Basic Nature of Salts
Determine whether aqueous solutions of the following salts are acidic, basic, or neutral:
(a) KBr
(b) NaHCO3
(c) Na2HPO4
(d) NH4F
Solution
Consider each of the ions separately in terms of its effect on the pH of the solution, as shown here:
(a) The K+ cation is inert (a Group I cation) and will not affect pH — it is “pH neutral”. The bromide ion is the conjugate base of a strong acid, and so it is of negligible base strength (no appreciable base ionization). The solution is neutral.
(b) The Na+ cation is pH neutral (a Group I cation) and will not affect the pH of the solution; while the HCO3– anion is amphiprotic. The Ka of HCO3– is 4.7 x 10−11,and its Kb is ![]()
Since Kb >> Ka, the solution is basic.
(c) The Na+ cation is a Group I cation, and will not affect the pH of the solution. Thehe ![]() anion is amphiprotic. The Ka of
anion is amphiprotic. The Ka of ![]() is 4.2 x 10−13,
is 4.2 x 10−13,
and its Kb is ![]() Because Kb >> Ka, the solution is basic.
Because Kb >> Ka, the solution is basic.
(d) The ![]() ion is acidic (see above discussion) and the F− ion is basic (conjugate base of the weak acid HF). Comparing the two ionization constants: Ka of
ion is acidic (see above discussion) and the F− ion is basic (conjugate base of the weak acid HF). Comparing the two ionization constants: Ka of ![]() is 5.6 x 10−10 and the Kb of F− is 1.4 x 10−11, so the solution is acidic, since Ka > Kb.
is 5.6 x 10−10 and the Kb of F− is 1.4 x 10−11, so the solution is acidic, since Ka > Kb.
Check Your Learning
Determine whether aqueous solutions of the following salts are acidic, basic, or neutral:
(a) K2CO3
(b) CaCl2
(c) KH2PO4
(d) (NH4)2CO3
The Ionization of Hydrated Metal Ions
Unlike the group 1 and 2 metal ions of the preceding examples (Na+, Ca2+, etc.), some metal ions function as acids in aqueous solutions. These ions are not just loosely solvated by water molecules when dissolved, instead they are covalently bonded to a fixed number of water molecules to yield a complex ion (see chapter on coordination chemistry). As an example, the dissolution of aluminum nitrate in water is typically represented as
However, the aluminum(III) ion actually reacts with six water molecules to form a stable complex ion, and so the more explicit representation of the dissolution process is
Al(NO3)(s) + 6H2O(l) ![]() Al(H2O)63+(aq) + 3NO3–(aq)
Al(H2O)63+(aq) + 3NO3–(aq)
As shown in Figure 95-1, the Al(H2O)63+ ions involve bonds between a central Al atom and the O atoms of the six water molecules. Consequently, the bonded water molecules’ O–H bonds are more polar than in nonbonded water molecules, making the bonded molecules more prone to donation of a hydrogen ion:
The conjugate base produced by this process contains five other bonded water molecules capable of acting as acids, and so the sequential or step-wise transfer of protons is possible as depicted in few equations below:
![A reaction is shown using ball and stick models. On the left, inside brackets with a superscript of 3 plus outside to the right is structure labeled “[ A l ( H subscript 2 O ) subscript 6 ] superscript 3 plus.” Inside the brackets is s central grey atom to which 6 red atoms are bonded in an arrangement that distributes them evenly about the central grey atom. Each red atom has two smaller white atoms attached in a forked or bent arrangement. Outside the brackets to the right is a space-filling model that includes a red central sphere with two smaller white spheres attached in a bent arrangement. Beneath this structure is the label “H subscript 2 O.” A double sided arrow follows. Another set of brackets follows to the right of the arrows which have a superscript of two plus outside to the right. The structure inside the brackets is similar to that on the left, except a white atom is removed from the structure. The label below is also changed to “[ A l ( H subscript 2 O ) subscript 5 O H ] superscript 2 plus.” To the right of this structure and outside the brackets is a space filling model with a central red sphere to which 3 smaller white spheres are attached. This structure is labeled “H subscript 3 O superscript plus.”](https://pressbooks.openedmb.ca/app/uploads/sites/94/2024/05/CNX_Chem_14_04_hydronium-1.jpg)
FIGURE 95-1: When an aluminum ion reacts with water, the hydrated aluminum ion becomes a weak acid.
Aside from the alkali metals (group 1) and some alkaline earth metals (group 2), most other metal ions will undergo acid ionization to some extent when dissolved in water. The acid strength of these complex ions typically increases with increasing charge and decreasing size of the metal ions. The first-step acid ionization equations for a few other acidic metal ions are shown below:
Hydrolysis of [Al(H2O)6]3+
Calculate the pH of a 0.10 M solution of aluminum chloride, which dissolves completely to give the hydrated aluminum ion [Al(H2O)6]3+ in aqueous solution.
Solution
The equation for the reaction and Ka are:
An ICE table with the provided information is
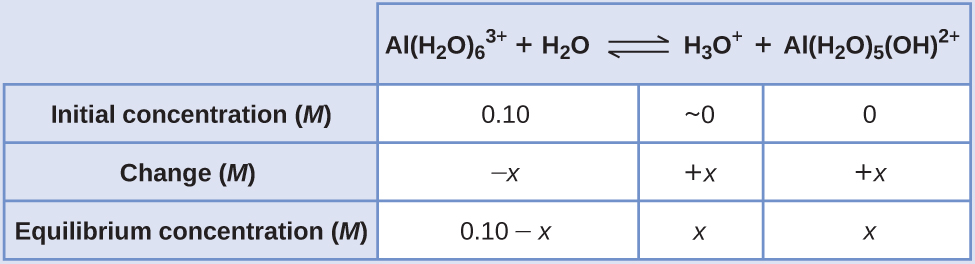
Substituting the expressions for the equilibrium concentrations into the equation for the ionization constant yields:
Assuming x << 0.10 and solving the simplified equation gives:
The ICE table defined x as equal to the hydronium ion concentration, and so the pH is calculated to be
Check Your Learning
What is ![]() in a 0.15 M solution of Al(NO3)3 that contains enough of the strong acid HNO3 to bring [H3O+] to 0.10 M?
in a 0.15 M solution of Al(NO3)3 that contains enough of the strong acid HNO3 to bring [H3O+] to 0.10 M?
Key Concepts and Summary
The ions composing salts may possess acidic or basic character, ionizing when dissolved in water to yield acidic or basic solutions. Acidic cations are typically the conjugate partners of weak bases, and basic anions are the conjugate partners of weak acids. Many metal ions bond to water molecules when dissolved to yield complex ions that may function as acids.
Section 95 Practice Problems
Click on this link to access the worked answers to these problems (as a downloadable pdf).
1. Determine whether aqueous solutions of the following salts are acidic, basic, or neutral:
(a) Al(NO3)3
(b) RbI
(c) KHCO2
(d) CH3NH3Br
2. Determine whether aqueous solutions of the following salts are acidic, basic, or neutral:
(a) FeCl3
(b) K2CO3
(c) NH4Br
(d) KClO4
Answer(s): (a) acidic; (b) basic; (c) acidic; (d) neutral
3. Determine whether the following 0.10 M aqueous solutions are acidic, basic or neutral. For each solution, indicate what the pH-determining reaction, the major species present, and the pH at 25 oC. Note that Ka and Kb values for the weak acids and bases are available in the Appendices to this text.
(a) ammonium chloride, NH4Cl
(b) hydrogen chloride, HCl
(c) lithium nitrite, LiNO2
(d) sodium hydroxide, NaOH
(e) barium hydroxide, Ba(OH)2
4. Determine whether the following 0.10 M aqueous solutions are acidic, basic or neutral. For each solution, indicate what the pH-determining reaction, the major species present, and the pH at 25 oC. Note that Ka and Kb values for the weak acids and bases are available in the Appendices to this text.
(a) sodium nitrate, NaNO3
(b) sodium benzoate, NaC6H5CO2)
(c) potassium fluoride, KF
(d) methylammonium chloride, CH3NH3Cl
(e) sodium cyanide, NaCN
5. Novocaine, C13H21O2N2Cl, is the salt of the base procaine and hydrochloric acid. The ionization constant for procaine is 7.0 x 10−6. Is a solution of novocaine acidic or basic? What are [H3O+], [OH−], and pH of a 2.0% solution by mass of novocaine, assuming that the density of the solution is 1.0 g mL-1.

