85 Equilibrium Constants
Learning Objectives
By the end of this section, you will be able to:
- Derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions
- Calculate values of reaction quotients and equilibrium constants, using concentrations and pressures
- Relate the magnitude of an equilibrium constant to properties of the chemical system
The status of a reversible reaction is conveniently assessed by evaluating its reaction quotient (Q). For a reversible reaction described by
the reaction quotient is derived directly from the stoichiometry of the balanced equation as
where the subscript c denotes the use of molar concentrations in the expression. If the reactants and products are gaseous, a reaction quotient may be similarly derived using partial pressures:
Note that the reaction quotient equations above are a simplification of more rigorous expressions that use relative values for concentrations and pressures rather than absolute values. Reaction quotients (and equilibrium constants, which we will discussed later in the chapter) should, technically, be defined in terms of the activity of a substance. The activity of a substance is its “effective concentration” in a reaction mixture.
- for substances that are dissolved in solution, the activity can be estimated as the ratio of the concentration of that substance (in M) divided by the standard concentration (in M).
-
- for a dilute (ideal) solution, the standard state is 1 M.
- for gasses, activities are frequently defined in terms of pressure: the activity can be estimated as the partial pressure of the gas divided by the standard pressure.
-
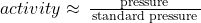
- for an ideal gas, the standard state is 1 bar (~1 atm or ~760 mm Hg).
-
- for substances that are liquids or solids, the activity is simply “1”.
- Under normal pressures, the “concentration” of the liquid or solid does not change, and the “amount” of the solid or liquid ddoes not affect the “concentration” (see Figure 85-1)
- even if a solute is dissolved in the liquid, the concentration of the liquid is largely unchanged.
- Thus, for example, the activity of liquid water (even if it has solutes dissolved in it) could be represented as follows:
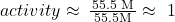
- Thus, for example, the activity of liquid water (even if it has solutes dissolved in it) could be represented as follows:
- Under extreme conditions (of pressure or concentration) correction factors need to be applied to obtain an accurate value of activity.

Since the units cancel out when activities are calculated, activities (and, thus, reaction quotients and equilibrium constants) are dimensionless numbers (have no units). Moreover, since the activities of liquids and solids are usually ≈ 1, they are ignored in the expressions for reaction quotients (and equilibrium constants).
Writing Reaction Quotient Expressions
Write the reaction quotient expression (Qc or Qp as indicated) for each of the following reactions:
(a) Qc for: ![]()
(b) Qc for: ![]()
(c) Qc for: ![]()
(d) Qc for: H2CO3(aq) ![]() CO2(aq) + H2O(l)
CO2(aq) + H2O(l)
(e) QP for: 2KClO3(s) ![]() 2KCl(s) + 3O2(g)
2KCl(s) + 3O2(g)
Solution
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
(e) QP = (PO2)3
Check Your Learning
Write the concentration-based reaction quotient expression for each of the following reactions:
(a) ![]()
(b) ![]()
(c) ![]()
(a) ![]() (b)
(b) ![]() (c)
(c) ![]()
The numerical value of Q varies as a reaction proceeds towards equilibrium; therefore, it can serve as a useful indicator of the reaction’s status. To illustrate this point, consider the oxidation of sulfur dioxide:
Two different experimental scenarios are depicted in Figure 85-2. In Panel (a), the reaction is initiated with the addition of a mixture of reactants only, SO2 and O2. In Panel (b), the reaction is initiated by the addition with only the “product”. The figure shows that, regardless of the direction of approach, equilibrium will ultimately be achieved.
![Four graphs are shown and labeled, “a,” “b,” “c,” and “d.” All four graphs have a vertical dotted line running through the middle labeled, “Equilibrium is reached.” The y-axis on graph a is labeled, “Concentration,” and the x-axis is labeled, “Time.” Three curves are plotted on graph a. The first is labeled, “[ S O subscript 2 ];” this line starts high on the y-axis, ends midway down the y-axis, has a steep initial slope and a more gradual slope as it approaches the far right on the x-axis. The second curve on this graph is labeled, “[ O subscript 2 ];” this line mimics the first except that it starts and ends about fifty percent lower on the y-axis. The third curve is the inverse of the first in shape and is labeled, “[ S O subscript 3 ].” The y-axis on graph b is labeled, “Concentration,” and the x-axis is labeled, “Time.” Three curves are plotted on graph b. The first is labeled, “[ S O subscript 2 ];” this line starts low on the y-axis, ends midway up the y-axis, has a steep initial slope and a more gradual slope as it approaches the far right on the x-axis. The second curve on this graph is labeled, “[ O subscript 2 ];” this line mimics the first except that it ends about fifty percent lower on the y-axis. The third curve is the inverse of the first in shape and is labeled, “[ S O subscript 3 ].” The y-axis on graph c is labeled, “Reaction Quotient,” and the x-axis is labeled, “Time.” A single curve is plotted on graph c. This curve begins at the bottom of the y-axis and rises steeply up near the top of the y-axis, then levels off into a horizontal line. The top point of this line is labeled, “k.” The y-axis on graph d is labeled, “Reaction Quotient,” and the x-axis is labeled, “Time.” A single curve is plotted on graph d. This curve begins near the edge of the top of the y-axis and falls steeply toward the x-axis, then levels off into a horizontal line. The bottom point of this line is labeled, “k.”](https://pressbooks.openedmb.ca/app/uploads/sites/94/2024/02/CNX_Chem_13_02_quotient-1.jpg)
As the reaction proceeds toward equilibrium in the forward direction, reactant concentrations decrease (as does the denominator of Qc), product concentration increases (as does the numerator of Qc), and the reaction quotient consequently increases. When equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of Qc.
If the reaction begins with only product present, the value of Qc is initially undefined (immeasurably large, or infinite):
In this case, the reaction proceeds toward equilibrium in the reverse direction. The product concentration and the numerator of Qc decrease with time, the reactant concentrations and the denominator of Qc increase, and the reaction quotient consequently decreases until it becomes constant at equilibrium.
Comparison of the data plots in Figure 85-2 shows that both experimental scenarios resulted in the same value for the equilibrium constant. This is a general observation for all equilibrium systems, known as the law of mass action: at a given temperature, the reaction quotient for a system at equilibrium is constant. The constant value of Q exhibited by a system at equilibrium is called the equilibrium constant, K:
Evaluating a Reaction Quotient
Gaseous nitrogen dioxide forms dinitrogen tetroxide according to this equation:
When 0.10 mol NO2 is added to a 1.0 L flask at 25 °C, the concentration changes so that at equilibrium, [NO2] = 0.016 M and [N2O4] = 0.042 M.
(a) What is the value of the reaction quotient before any reaction occurs?
(b) What is the value of the equilibrium constant for the reaction?
Solution
As for all equilibrium calculations in this text, use the simplified equations for Q and K and disregard any concentration or pressure units, as noted previously in this section.
(a) Before any product is formed, ![]() and [N2O4] = 0 M. Thus,
and [N2O4] = 0 M. Thus,
(b) At equilibrium, ![]() The equilibrium constant is 1.6 x 102.
The equilibrium constant is 1.6 x 102.
Check Your Learning
For the reaction ![]() the concentrations at equilibrium are [SO2] = 0.90 M, [O2] = 0.35 M, and [SO3] = 1.1 M. What is the value of the equilibrium constant, Kc?
the concentrations at equilibrium are [SO2] = 0.90 M, [O2] = 0.35 M, and [SO3] = 1.1 M. What is the value of the equilibrium constant, Kc?
Kc = 4.3
By its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be interpreted with regard to the extent of the forward reaction. A reaction exhibiting a large K will reach equilibrium when most of the reactant has been converted to product, whereas a small K indicates the reaction achieves equilibrium after very little reactant has been converted. It is important to keep in mind that the magnitude of K does not indicate how rapidly or slowly equilibrium will be reached. Some equilibria are established so quickly as to be nearly instantaneous, and others so slowly that no perceptible change is observed over the course of days, years, or longer.
The equilibrium constant for a reaction can be used to predict the behavior of mixtures containing its reactants and/or products. As demonstrated by the sulfur dioxide oxidation process described above, a chemical reaction will proceed in whatever direction is necessary to achieve equilibrium. Comparing Q to K for an equilibrium system of interest allows prediction of the net direction of reaction.
To further illustrate this important point, consider the reversible reaction shown below:
The bar charts in Figure 85-3 represent changes in reactant and product concentrations for three different reaction mixtures. The reaction quotients for mixtures 1 and 3 are initially lesser than the reaction’s equilibrium constant, so each of these mixtures will experience a net forward reaction to achieve equilibrium. The reaction quotient for mixture 2 is initially greater than the equilibrium constant, so this mixture will proceed in the reverse direction until equilibrium is established.
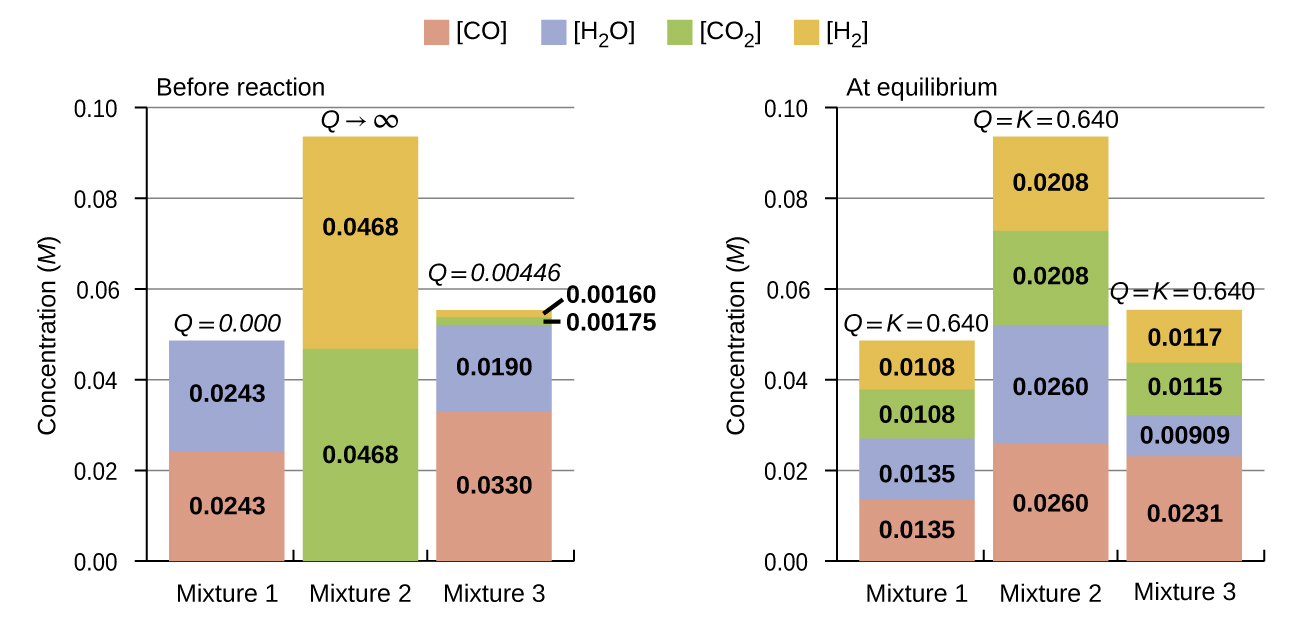
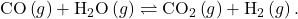
Predicting the Direction of Reaction
Given here are the starting concentrations of reactants and products for three experiments involving this reaction:
Determine in which direction the reaction proceeds as it goes to equilibrium in each of the three experiments shown.
| Reactants/Products | Experiment 1 | Experiment 2 | Experiment 3 |
|---|---|---|---|
| [CO]i | 0.020 M | 0.011 M | 0.0094 M |
| [H2O]i | 0.020 M | 0.0011 M | 0.0025 M |
| [CO2]i | 0.0040 M | 0.037 M | 0.0015 M |
| [H2]i | 0.0040 M | 0.046 M | 0.0076 M |
Solution
Experiment 1:
Qc < Kc (0.040 < 0.64)
The reaction will proceed in the forward direction.
Experiment 2:
Qc > Kc (140 > 0.64)
The reaction will proceed in the reverse direction.
Experiment 3:
Qc < Kc (0.48 < 0.64)
The reaction will proceed in the forward direction.
Check Your Learning
Calculate the reaction quotient and determine the direction in which each of the following reactions will proceed to reach equilibrium.
(a) A 1.00 L flask containing 0.0500 mol of NO(g), 0.0155 mol of Cl2(g), and 0.500 mol of NOCl(g):
(b) A 5.0 L flask containing 17 g of NH3(g), 14 g of N2(g), and 12 g of H2 (g):
(c) A 2.00 L flask containing 230 g of SO3(g):
(a) Qc = 6.45 x 103, forward. (b) Qc = 0.23, reverse. (c) Qc = 0, forward.
Homogeneous Equilibria
A homogeneous equilibrium is one in which all reactants and products (and any catalysts, if applicable) are present in the same phase. By this definition, homogeneous equilibria take place in solutions. These solutions are most commonly either liquid or gaseous phases, as shown by the examples below:
![Rendered by QuickLaTeX.com \begin{array}{cccccccc}\hfill {\text{C}}_{2}{\text{H}}_{2}\left(aq\right)+2{\text{Br}}_{2}\left(aq\right)& \rightleftharpoons\hfill & {\text{C}}_{2}{\text{H}}_{2}{\text{Br}}_{4}\left(aq\right)\hfill & & & \hfill {K}_{c}& =\hfill & \frac{\left[{\text{C}}_{2}{\text{H}}_{2}{\text{Br}}_{4}\right]}{\left[{\text{C}}_{2}{\text{H}}_{2}\right]\phantom{\rule{0.2em}{0ex}}{\left[{\text{Br}}_{2}\right]}^{2}}\hfill \\ \hfill {\text{I}}_{2}\left(aq\right)+{\text{I}}^{\text{−}}\left(aq\right)& \rightleftharpoons\hfill & {\text{I}}_{3}{}^{\text{−}}\left(aq\right)\hfill & & & \hfill {K}_{c}& =\hfill & \frac{\left[{\text{I}}_{3}{}^{\text{−}}\right]}{\left[{\text{I}}_{2}\right]\left[{\text{I}}^{\text{−}}\right]}\hfill \\ \hfill \text{HF}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)& \rightleftharpoons\hfill & {\text{H}}_{3}{\text{O}}^{\text{+}}\left(aq\right)+{\text{F}}^{\text{−}}\left(aq\right)\hfill & & & \hfill {K}_{c}& =\hfill & \frac{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\left[{\text{F}}^{\text{−}}\right]}{\left[\text{HF}\right]}\hfill \\ \hfill {\text{NH}}_{3}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)& \rightleftharpoons\hfill & {\text{NH}}_{4}{}^{\text{+}}\left(aq\right)+{\text{OH}}^{\text{−}}\left(aq\right)\hfill & & & \hfill {K}_{c}& =\hfill & \frac{\left[{\text{NH}}_{4}{}^{\text{+}}\right]\left[{\text{OH}}^{\text{−}}\right]}{\left[{\text{NH}}_{3}\right]}\hfill \end{array}](https://pressbooks.openedmb.ca/app/uploads/quicklatex/quicklatex.com-b28fc02fa2b1de92d3a04075b02aee98_l3.png)
These examples all involve aqueous solutions, those in which water functions as the solvent. In the last two examples, water also functions as a reactant, but its concentration is not included in the reaction quotient. The reason for this omission is related to the more rigorous form of the Q (or K) expression mentioned previously in this chapter, in which relative concentrations for liquids and solids are equal to 1 and needn’t be included. Consequently, reaction quotients include concentration or pressure terms only for gaseous and solute species.
The equilibria below all involve gas-phase solutions:
![Rendered by QuickLaTeX.com \begin{array}{cccccccc}\hfill {\text{C}}_{2}{\text{H}}_{6}\left(g\right)& \rightleftharpoons\hfill & {\text{C}}_{2}{\text{H}}_{4}\left(g\right)+{\text{H}}_{2}\left(g\right)\hfill & & & \hfill {K}_{c}& =\hfill & \frac{\left[{\text{C}}_{2}{\text{H}}_{4}\right]\phantom{\rule{0.2em}{0ex}}\left[{\text{H}}_{2}\right]}{\left[{\text{C}}_{2}{\text{H}}_{6}\right]}\hfill \\ \hfill 3{\text{O}}_{2}\left(g\right)& \rightleftharpoons\hfill & 2{\text{O}}_{3}\left(g\right)\hfill & & & \hfill {K}_{c}& =\hfill & \frac{{\left[{\text{O}}_{3}\right]}^{2}}{{\left[{\text{O}}_{2}\right]}^{3}}\hfill \\ \hfill {\text{N}}_{2}\left(g\right)+3{\text{H}}_{2}\left(g\right)& \rightleftharpoons\hfill & 2{\text{NH}}_{3}\left(g\right)\hfill & & & \hfill {K}_{c}& =\hfill & \frac{{\left[{\text{NH}}_{3}\right]}^{2}}{\left[{\text{N}}_{2}\right]{\phantom{\rule{0.2em}{0ex}}\left[{\text{H}}_{2}\right]}^{3}}\hfill \\ \hfill {\text{C}}_{3}{\text{H}}_{8}\left(g\right)+5{\text{O}}_{2}\left(g\right)&\rightleftharpoons\hfill & 3{\text{CO}}_{2}\left(g\right)+4{\text{H}}_{2}\text{O}\left(g\right)\hfill & & & \hfill {K}_{c}& =\hfill & \frac{{\left[{\text{CO}}_{2}\right]}^{3}{\left[{\text{H}}_{2}\text{O}\right]}^{4}}{\left[{\text{C}}_{3}{\text{H}}_{8}\right]{\left[{\text{O}}_{2}\right]}^{5}}\hfill \end{array}](https://pressbooks.openedmb.ca/app/uploads/quicklatex/quicklatex.com-2e82e40c76367e6a70882e85f93b578b_l3.png)
For gas-phase solutions, the equilibrium constant may be expressed in terms of either the molar concentrations (Kc) or partial pressures (Kp) of the reactants and products. A relation between these two K values may be simply derived from the ideal gas equation and the definition of molarity:
where P is partial pressure, V is volume, n is molar amount, R is the gas constant, T is temperature, and M is molar concentration.
For the gas-phase reaction ![]()
And so, the relationship between Kc and KP is
where Δn is the difference in the molar amounts of product and reactant gases, in this case:
Calculation of KP
Write the equations relating Kc to KP for each of the following reactions:
(a) ![]()
(b) ![]()
(c) ![]()
(d) Kc is equal to 0.28 for the following reaction at 900 °C:
What is KP at this temperature?
Solution
(a) Δn = (2) − (1) = 1
KP = Kc (RT)Δn = Kc (RT)1 = Kc (RT)
(b) Δn = (2) − (2) = 0
KP = Kc (RT)Δn = Kc (RT)0 = Kc
(c) Δn = (2) − (1 + 3) = −2
KP = Kc (RT)Δn = Kc (RT)−2 = ![]()
(d) KP = Kc (RT)Δn = (0.28)[(0.0821)(1173)]−2 = 3.0 x 10−5
Check Your Learning
Write the equations relating Kc to KP for each of the following reactions:
(a) ![]()
(b) ![]()
(c) ![]()
(d) At 227 °C, the following reaction has Kc = 0.0952:
What would be the value of KP at this temperature?
(a) KP = Kc (RT)−1; (b) KP = Kc (RT); (c) KP = Kc (RT); (d) 160 or 1.6 x 102
Heterogeneous Equilibria
A heterogeneous equilibrium involves reactants and products in two or more different phases, as illustrated by the following examples:
Recall that species that are solids and liquids are not included in the equilibrium expression, as discussed previously.
Coupled Equilibria
The equilibrium systems discussed so far have all been relatively simple, involving just single reversible reactions. Many systems, however, involve two or more coupled equilibrium reactions, those which have in common one or more reactant or product species. Since the law of mass action allows for a straightforward derivation of equilibrium constant expressions from balanced chemical equations, the K value for a system involving coupled equilibria can be related to the K values of the individual reactions. Three basic manipulations are involved in this approach, as described below.
1. Changing the direction of a chemical equation essentially swaps the identities of “reactants” and “products,” and so the equilibrium constant for the reversed equation is simply the reciprocal of that for the forward equation.
![Rendered by QuickLaTeX.com \begin{array}{}\\ \\ \\ \text{A}\rightleftharpoons\text{B}\phantom{\rule{5em}{0ex}}{\text{K}}_{\text{c}}=\frac{\left[\text{B}\right]}{\left[\text{A}\right]}\hfill \\ \text{B}\rightleftharpoons\text{A}\phantom{\rule{5em}{0ex}}{\text{K}}_{\text{c'}}=\frac{\left[\text{A}\right]}{\left[\text{B}\right]}\hfill \end{array}](https://pressbooks.openedmb.ca/app/uploads/quicklatex/quicklatex.com-a4ca63bd05d60ee378106b421e388e6a_l3.png)
2. Changing the stoichiometric coefficients in an equation by some factor x results in an exponential change in the equilibrium constant by that same factor:
![Rendered by QuickLaTeX.com \begin{array}{}\\ \\ \\ \text{A}\rightleftharpoons\text{B}\phantom{\rule{6em}{0ex}}{\text{K}}_{\text{c}}=\frac{\left[\text{B}\right]}{\left[\text{A}\right]}\hfill \\ \text{xA}\rightleftharpoons\text{xB}\phantom{\rule{5em}{0ex}}{\text{K}}_{\text{c'}}=\frac{\left[\text{B}{\right]}^{\text{x}}}{\left[\text{A}]^{\text{x}{\right}}}\hfill \end{array}](https://pressbooks.openedmb.ca/app/uploads/quicklatex/quicklatex.com-a4ff6d78f5a1e65abb0a79c2b8f04dc1_l3.png)
3. Adding two or more equilibrium equations together yields an overall equation whose equilibrium constant is the mathematical product of the individual reaction’s K values:
![Rendered by QuickLaTeX.com \begin{array}{}\\ \\ \\ \text{A}\rightleftharpoons\text{B}\phantom{\rule{5em}{0ex}}{\text{K}}_{\text{c1}}=\frac{\left[\text{B}\right]}{\left[\text{A}\right]}\hfill \\ \text{B}\rightleftharpoons\text{C}\phantom{\rule{5em}{0ex}}{\text{K}}_{\text{c2}}=\frac{\left[\text{C}\right]}{\left[\text{B}\right]}\hfill \end{array}](https://pressbooks.openedmb.ca/app/uploads/quicklatex/quicklatex.com-72d266d44b5d093c80b74f496baf4379_l3.png)
The net reaction for these coupled equilibria is obtained by summing the two equilibrium equations and canceling any redundancies:
![Rendered by QuickLaTeX.com \begin{array}{l}\text{A}+\text{B}\rightleftharpoons\text{B}+\text{C}\\ \\ \text{A}\rightleftharpoons\text{C}\phantom{\rule{5em}{0ex}}{\text{K}}_{\text{c'}}=\frac{\left[\text{C}\right]}{\left[\text{A}\right]}\end{array}](https://pressbooks.openedmb.ca/app/uploads/quicklatex/quicklatex.com-eb7e7a79976a7acc644fa8ea91f6b45a_l3.png)
Comparing the equilibrium constant for the net reaction to those for the two coupled equilibrium reactions reveals the following relationship:
Equilibrium Constants for Coupled Reactions
A mixture containing nitrogen, hydrogen, and iodine established the following equilibrium at 400 °C:
Use the information below to calculate Kc for this reaction.
Solution
The equilibrium equation of interest and its K value may be derived from the equations for the two coupled reactions as follows.
Reverse the first coupled reaction equation:
Multiply the second coupled reaction by 3:
Finally, add the two revised equations:
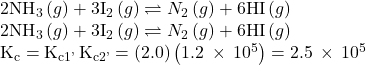
Check Your Learning
Use the provided information to calculate Kc for the following reaction at 550 °C:
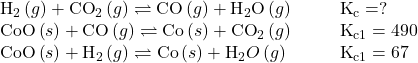
Kc = 0.14
Key Concepts and Summary
The composition of a reaction mixture may be represented by a mathematical function known as the reaction quotient, Q. For a reaction at equilibrium, the composition is constant, and Q is called the equilibrium constant, K.
A homogeneous equilibrium is an equilibrium in which all components are in the same phase. A heterogeneous equilibrium is an equilibrium in which components are in two or more phases.
In a dynamic equilibrium, the rate of the forward reaction is equal to the rate of the reverse reaction: the net rate of the reaction is zero.
Key Equations
-
For a reversible reaction described by
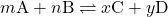
the reaction quotient is derived directly from the stoichiometry of the balanced equation as
![Rendered by QuickLaTeX.com {Q}_{c}=\phantom{\rule{0.2em}{0ex}}\frac{{\left[\text{C}\right]}^{x}\left[\text{D}{\right]}^{y}}{{\left[\text{A}\right]}^{m}\left[\text{B}]}^{n}}](https://pressbooks.openedmb.ca/app/uploads/quicklatex/quicklatex.com-93c35faba9a9d4ead3aa804f6c533f97_l3.png)
- If the reactants and products in the reaction above are gaseous, a reaction quotient may be similarly derived using partial pressures:
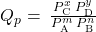
- Kc = Qc at equilibrium
- Kp = Qp at equilibrium
- KP = Kc (RT)Δn
Section 85 Practice Problems
Click on this link in order to view the worked answers to the problems in this section (as a downloadable pdf).
- Explain why there may be an infinite number of values for the reaction quotient of a reaction at a given temperature but there can be only one value for the equilibrium constant at that temperature.
2. Explain why an equilibrium between Br2(l) and Br2(g) would not be established if the container were not a closed vessel shown in Figure 85-3.
Answer(s): Equilibrium cannot be established between the liquid and the gas phase if the top is removed from the bottle because the system is not closed; one of the components of the equilibrium, the Br2 vapor, would escape from the bottle until all liquid disappeared. Thus, more liquid would evaporate than can condense back from the gas phase to the liquid phase.
3. If you observe the following reaction at equilibrium, is it possible to tell whether the reaction started with pure NO2 or with pure N2O4?
![]()
4. Write the mathematical expression for the reaction quotient, Qc, for each of the following reactions:
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
(e) ![]()
(f) ![]()
(g) ![]()
(h) ![]()
Answer(s): (a) ![]() (b)
(b) ![]() (c)
(c) ![]() (d) Qc = [SO2]; (e)
(d) Qc = [SO2]; (e) ![]() (f)
(f) ![]() (g)
(g) ![]() (h) Qc = [H2O]5
(h) Qc = [H2O]5
5. Write the mathematical expression for the reaction quotient, Qc, for each of the following reactions:
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
(e) ![]()
(f) ![]()
(g) ![]()
(h) ![]()
6. The initial concentrations or pressures of reactants and products are given for each of the following systems. Calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium.
(a) ![]() [NH3] = 0.20 M, [N2] = 1.00 M, [H2] = 1.00 M
[NH3] = 0.20 M, [N2] = 1.00 M, [H2] = 1.00 M
(b) ![]() NH3 = 3.0 atm, N2 = 2.0 atm, H2 = 1.0 atm
NH3 = 3.0 atm, N2 = 2.0 atm, H2 = 1.0 atm
(c) ![]() [SO3] = 0.00 M, [SO2] = 1.00 M, [O2] = 1.00 M
[SO3] = 0.00 M, [SO2] = 1.00 M, [O2] = 1.00 M
(d) ![]() SO3 = 1.00 atm, SO2 = 1.00 atm, O2 = 1.00 atm
SO3 = 1.00 atm, SO2 = 1.00 atm, O2 = 1.00 atm
(e) ![]() [NO] = 1.00 M, [Cl2] = 1.00 M, [NOCl] = 0 M
[NO] = 1.00 M, [Cl2] = 1.00 M, [NOCl] = 0 M
(f) ![]() NO = 10.0 atm, N2 = O2 = 5 atm
NO = 10.0 atm, N2 = O2 = 5 atm
Answer(s): (a) Qc 25 proceeds left; (b) QP 0.22 proceeds right; (c) Qc undefined proceeds left; (d) QP 1.00 proceeds right; (e) QP 0 proceeds right; (f) Qc 4 proceeds left
7. The initial concentrations or pressures of reactants and products are given for each of the following systems. Calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium.
(a) ![]() [NH3] = 0.50 M, [N2] = 0.15 M, [H2] = 0.12 M
[NH3] = 0.50 M, [N2] = 0.15 M, [H2] = 0.12 M
(b) ![]() NH3 = 2.00 atm, N2 = 10.00 atm, H2 = 10.00 atm
NH3 = 2.00 atm, N2 = 10.00 atm, H2 = 10.00 atm
(c) ![]() [SO3] = 2.00 M, [SO2] = 2.00 M, [O2] = 2.00 M
[SO3] = 2.00 M, [SO2] = 2.00 M, [O2] = 2.00 M
(d) ![]() SO2 = 1.00 atm, O2 = 1.130 atm, SO3 = 0 atm
SO2 = 1.00 atm, O2 = 1.130 atm, SO3 = 0 atm
(e) ![]() NO = 1.00 atm, Cl2 = 1.00 atm, NOCl = 0 atm
NO = 1.00 atm, Cl2 = 1.00 atm, NOCl = 0 atm
(f) ![]() [N2] = 0.100 M, [O2] = 0.200 M, [NO] = 1.00 M
[N2] = 0.100 M, [O2] = 0.200 M, [NO] = 1.00 M
8. Benzene is one of the compounds used as octane enhancers in unleaded gasoline. It is manufactured by the catalytic conversion of acetylene to benzene: ![]() Which value of Kc would make this reaction most useful commercially? Kc ≈ 0.01, Kc ≈ 1, or Kc ≈ 10. Explain your answer.
Which value of Kc would make this reaction most useful commercially? Kc ≈ 0.01, Kc ≈ 1, or Kc ≈ 10. Explain your answer.
Answer(s): Since ![]() a value of Kc ≈ 10 means that C6H6 predominates over C2H2. In such a case, the reaction would be commercially feasible if the rate to equilibrium is suitable.
a value of Kc ≈ 10 means that C6H6 predominates over C2H2. In such a case, the reaction would be commercially feasible if the rate to equilibrium is suitable.
9. The following reaction has KP = 4.50 x 10−5 at 720 K.
![]()
If a reaction vessel is filled with each gas to the partial pressures listed, in which direction will it shift to reach equilibrium? P(NH3) = 93 atm, P(N2) = 48 atm, and P(H2) = 52 atm
Answer(s): The system will shift toward the reactants to reach equilibrium.
10. Determine if the following system is at equilibrium. If not, in which direction will the system need to shift to reach equilibrium?
![]()
Answer(s): [SO2Cl2] = 0.12 M, [Cl2] = 0.16 M and [SO2] = 0.050 M. Kc for the reaction is 0.078.
11. Among the solubility rules previously discussed is the statement: All chlorides are soluble except Hg2Cl2, AgCl, PbCl2, and CuCl.
(a) Write the expression for the equilibrium constant for the reaction represented by the equation ![]() Is Kc > 1, < 1, or ≈ 1? Explain your answer.
Is Kc > 1, < 1, or ≈ 1? Explain your answer.
(b) Write the expression for the equilibrium constant for the reaction represented by the equation ![]() Is Kc > 1, < 1, or ≈ 1? Explain your answer.
Is Kc > 1, < 1, or ≈ 1? Explain your answer.
Answer(s): (a) Kc = [Ag+][Cl−] < 1. AgCl is insoluble; thus, the concentrations of ions are much less than 1 M; (b) ![]() > 1 because PbCl2 is insoluble and formation of the solid will reduce the concentration of ions to a low level (<1 M).
> 1 because PbCl2 is insoluble and formation of the solid will reduce the concentration of ions to a low level (<1 M).
12. Among the solubility rules previously discussed is the statement: Carbonates, phosphates, borates, and arsenates—except those of the ammonium ion and the alkali metals—are insoluble.
(a) Write the expression for the equilibrium constant for the reaction represented by the equation ![]() Is Kc > 1, < 1, or ≈ 1? Explain your answer.
Is Kc > 1, < 1, or ≈ 1? Explain your answer.
(b) Write the expression for the equilibrium constant for the reaction represented by the equation ![]() Is Kc > 1, < 1, or ≈ 1? Explain your answer.
Is Kc > 1, < 1, or ≈ 1? Explain your answer.
13. For a titration to be effective, the reaction must be rapid and the yield of the reaction must essentially be 100%. Is Kc > 1, < 1, or ≈ 1 for a titration reaction?
Answer(s): Kc > 1
14. For a precipitation reaction to be useful in a gravimetric analysis, the product of the reaction must be insoluble. Is Kc > 1, < 1, or ≈ 1 for a useful precipitation reaction?
15. Convert the values of Kc to values of KP or the values of KP to values of Kc.
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
Answer(s): (a) KP = 1.6 x 10−4; (b) KP = 50.2; (c) Kc = 5.34 x 10−39; (d) Kc = 4.60 x 10−3
16. Convert the values of Kc to values of KP or the values of KP to values of Kc.
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
Glossary
- equilibrium constant (K)
- value of the reaction quotient for a system at equilibrium; may be expressed using concentrations (Kc) or partial pressures (Kp)
- heterogeneous equilibria
- equilibria in which reactants and products occupy two or more different phases
- homogeneous equilibria
- equilibria in which all reactants and products occupy the same phase
- law of mass action
- when a reversible reaction has attained equilibrium at a given temperature, the reaction quotient remains constant
- reaction quotient (Q)
- mathematical function describing the relative amounts of reactants and products in a reaction mixture; may be expressed in terms of concentrations (Qc) or pressures (Qp)

