82 Catalysis
Learning Objectives
By the end of this section, you will be able to:
- Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams
- List examples of catalysis in natural and industrial processes
Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can increase the reaction rate without being consumed in the reaction. The concepts introduced in the previous section on reaction mechanisms provide the basis for understanding how catalysts are able to accomplish this very important function.
Figure 82-1 shows the reaction energy diagrams for a chemical process in the presence and absence of a catalyst. Inspection of the diagrams reveals several traits of these reactions. Consistent with the fact that the two diagrams represent the same overall reaction, both curves begin and end at the same energies. However, the uncatalyzed reaction proceeds with a notably lower activation energy. This difference illustrates the means by which a catalyst increases the rate of reaction: by providing an alternative reaction mechanism with a lower activation energy barrier. This increases the rate constant for the reaction and, thus, increases the rate of reaction. Although the catalyzed reaction mechanism for a reaction needn’t necessarily involve the same number of steps than the uncatalyzed mechanism, it must provide a reaction path whose rate determining step is faster (lower Ea).
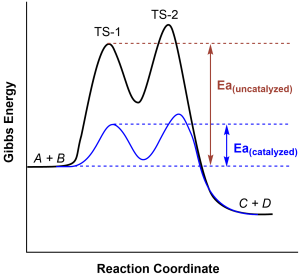
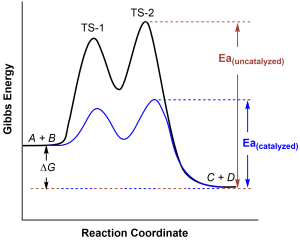
It is important to note that the presence of a catalyst cannot affect the spontaneity of reaction. As can be seen in Figure 82-2, the energy difference between the reactants (A + B) and the products (C + D) is unchanged by the presence of a catalyst, since ΔE (ΔG) is a state function. However, the activation energy barriers for the reverse reaction (C + D → A + B) are also lower relative to the uncatalyzed reverse reaction. Thus the catalyst lowers the activation energy barrier for both the forward and reverse reactions: the rate of both reactions is increased.
Reaction Diagrams for Catalyzed Reactions
The two reaction diagrams here represent the same reaction: one without a catalyst and one with a catalyst. Estimate the activation energy for each process, and identify which one involves a catalyst.
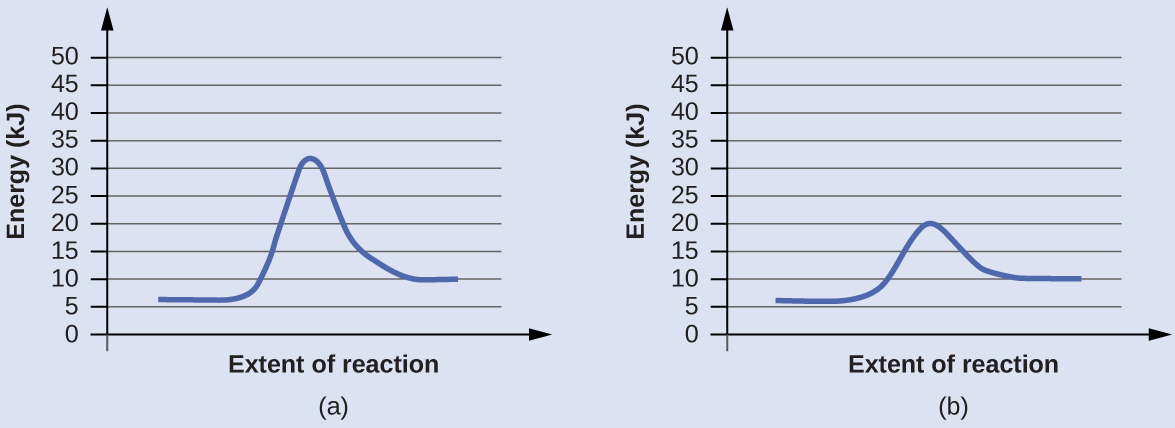
Solution
Activation energies are calculated by subtracting the reactant energy from the transition state energy.
The catalyzed reaction is the one with lesser activation energy, in this case represented by diagram b.
Check Your Learning
Reaction diagrams for a chemical process with and without a catalyst are shown below. Both reactions involve a two-step mechanism with a rate-determining first step. Compute activation energies for the first step of each mechanism, and identify which corresponds to the catalyzed reaction. How do the second steps of these two mechanisms compare?
Homogeneous Catalysts
A homogeneous catalyst is present in the same phase as the reactants. It interacts with a reactant to form an intermediate substance, which then decomposes or reacts with another reactant in one or more steps to regenerate the original catalyst and form product.
As an important illustration of homogeneous catalysis, consider the earth’s ozone layer. Ozone in the upper atmosphere, which protects the earth from ultraviolet radiation, is formed when oxygen molecules absorb ultraviolet light and undergo the reaction:
Ozone is a relatively unstable molecule that decomposes to yield diatomic oxygen by the reverse of this equation. This decomposition reaction is consistent with the following two-step mechanism:
A number of substances can catalyze the decomposition of ozone. For example, the nitric oxide–catalyzed decomposition of ozone is believed to occur via the following three-step mechanism:
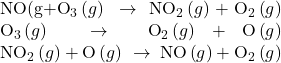
As required, the overall reaction is the same for both the two-step uncatalyzed mechanism and the three-step NO-catalyzed mechanism:
Notice that NO is a reactant in the first step of the mechanism and a product in the last step. This is another characteristic trait of a catalyst: Though it participates in the chemical reaction, it is not consumed by the reaction.
The 1995 Nobel Prize in Chemistry was shared by Paul J. Crutzen, Mario J. Molina, pictured in Figure 82-3, and F. Sherwood Rowland “for their work in atmospheric chemistry, particularly concerning the formation and decomposition of ozone.”1 Molina, a Mexican citizen, carried out the majority of his work at the Massachusetts Institute of Technology (MIT).
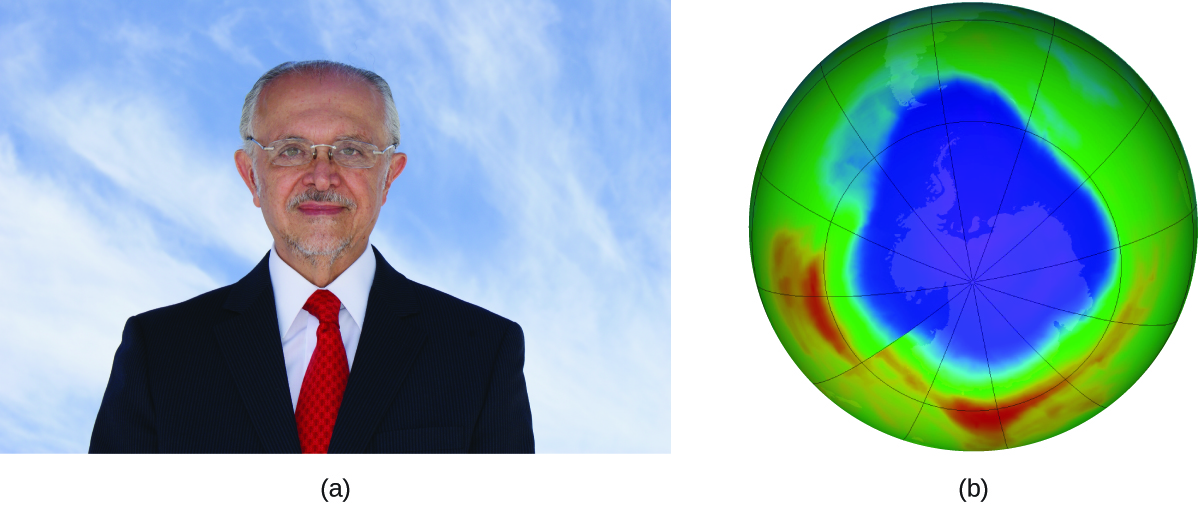
In 1974, Molina and Rowland published a paper in the journal Nature detailing the threat of chlorofluorocarbon gases to the stability of the ozone layer in earth’s upper atmosphere. The ozone layer protects earth from solar radiation by absorbing ultraviolet light. As chemical reactions deplete the amount of ozone in the upper atmosphere, a measurable “hole” forms above Antarctica, and an increase in the amount of solar ultraviolet radiation— strongly linked to the prevalence of skin cancers—reaches earth’s surface. The work of Molina and Rowland was instrumental in the adoption of the Montreal Protocol, an international treaty signed in 1987 that successfully began phasing out production of chemicals linked to ozone destruction.
Molina and Rowland demonstrated that chlorine atoms from human-made chemicals can catalyze ozone destruction in a process similar to that by which NO accelerates the depletion of ozone. Chlorine atoms are generated when chlorocarbons or chlorofluorocarbons—once widely used as refrigerants and propellants—are photochemically decomposed by ultraviolet light or react with hydroxyl radicals. A sample mechanism is shown here using methyl chloride:
Chlorine radicals break down ozone and are regenerated by the following catalytic cycle:
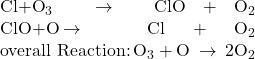
A single monatomic chlorine can break down thousands of ozone molecules. Luckily, the majority of atmospheric chlorine exists as the catalytically inactive forms Cl2 and ClONO2.
Since receiving his portion of the Nobel Prize, Molina has continued his work in atmospheric chemistry at MIT.
APPLICATION – Enzymes – Biological Catalysts
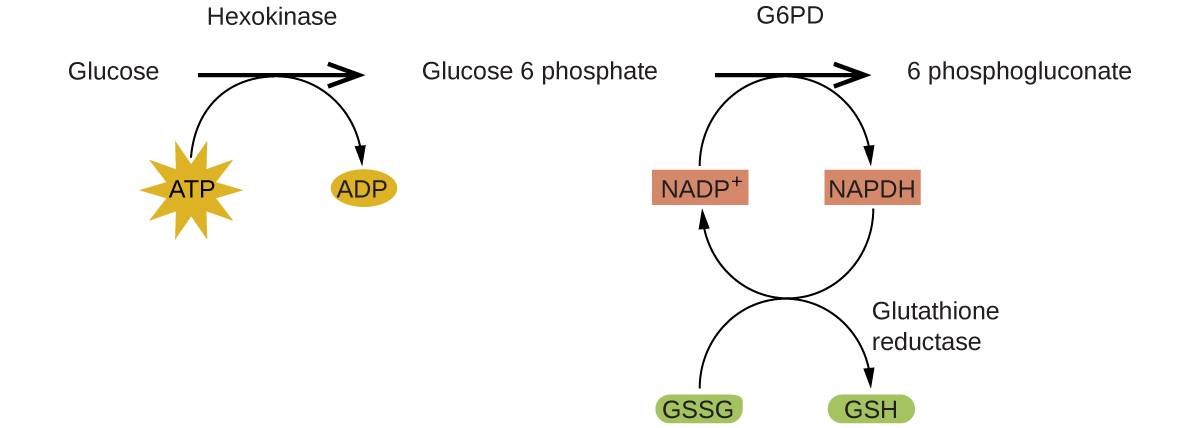
Enzymes in the human body act as catalysts for important chemical reactions in cellular metabolism. As such, a deficiency of a particular enzyme can translate to a life-threatening disease. G6PD (glucose-6-phosphate dehydrogenase) deficiency, a genetic condition that results in a shortage of the enzyme glucose-6-phosphate dehydrogenase, is the most common enzyme deficiency in humans. This enzyme, shown in Figure 82-4, is the rate-limiting enzyme for the metabolic pathway that supplies NADPH to cells (see Figure 82-5).
A disruption in this pathway can lead to reduced glutathione in red blood cells; once all glutathione is consumed, enzymes and other proteins such as hemoglobin are susceptible to damage. For example, hemoglobin can be metabolized to bilirubin, which leads to jaundice, a condition that can become severe. People who suffer from G6PD deficiency must avoid certain foods and medicines containing chemicals that can trigger damage their glutathione-deficient red blood cells.
Heterogeneous Catalysts
A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. Such catalysts generally function by furnishing an active surface upon which a reaction can occur. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid phase.
Heterogeneous catalysis typically involves the following processes:
- Adsorption of the reactant(s) onto the surface of the catalyst
- Activation of the adsorbed reactant(s)
- Reaction of the adsorbed reactant(s)
- Desorption of product(s) from the surface of the catalyst
Figure 82-6 illustrates the steps of a mechanism for the reaction of compounds containing a carbon–carbon double bond with hydrogen on a nickel catalyst. Nickel is the catalyst used in the hydrogenation of polyunsaturated fats and oils (which contain several carbon–carbon double bonds) to produce saturated fats and oils (which contain only carbon–carbon single bonds).
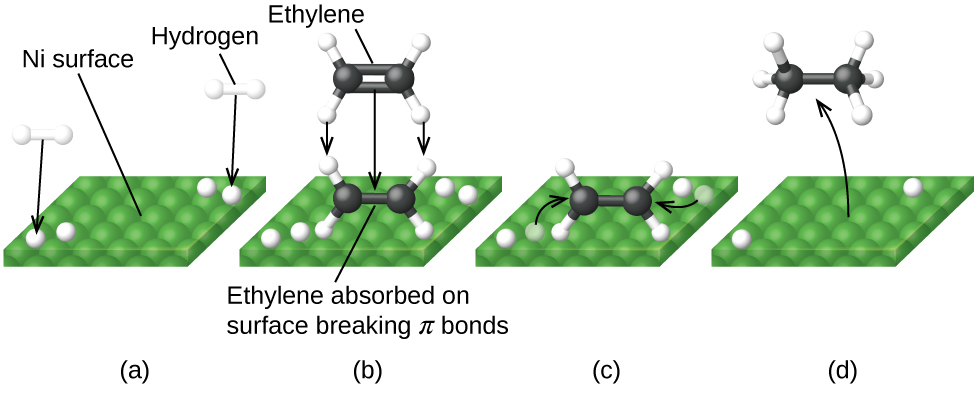
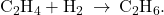 (a) Hydrogen is adsorbed on the surface, breaking the H–H bonds and forming Ni–H bonds. (b) Ethylene is adsorbed on the surface, breaking the C–C π-bond and forming Ni–C bonds. (c) Atoms diffuse across the surface and form new C–H bonds when they collide. (d) C2H6 molecules desorb from the Ni surface.
(a) Hydrogen is adsorbed on the surface, breaking the H–H bonds and forming Ni–H bonds. (b) Ethylene is adsorbed on the surface, breaking the C–C π-bond and forming Ni–C bonds. (c) Atoms diffuse across the surface and form new C–H bonds when they collide. (d) C2H6 molecules desorb from the Ni surface.
Many important chemical products are prepared via industrial processes that use heterogeneous catalysts, including ammonia, nitric acid, sulfuric acid, and methanol. Heterogeneous catalysts are also used in the catalytic converters found on most gasoline-powered automobiles, shown in Figure 82-7. Scientists developed catalytic converters to reduce the amount of toxic emissions produced by burning gasoline in internal combustion engines. By utilizing a carefully selected blend of catalytically active metals, it is possible to effect complete combustion of all carbon-containing compounds to carbon dioxide while also reducing the output of nitrogen oxides. This is particularly impressive when we consider that one step involves adding more oxygen to the molecule and the other involves removing the oxygen. A catalytic converter allows for the combustion of all carbon-containing compounds to carbon dioxide, while at the same time reducing the output of nitrogen oxide and other pollutants in emissions from gasoline-burning engines.
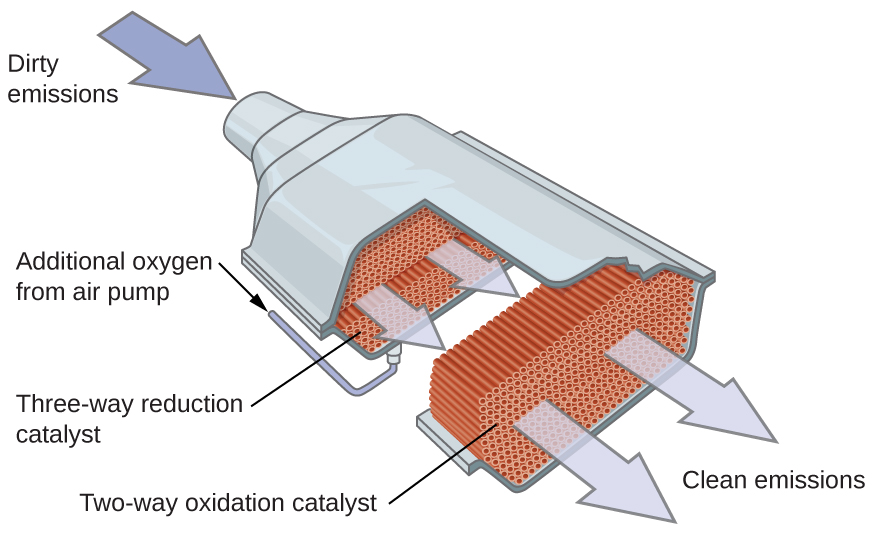
Most modern, three-way catalytic converters possess a surface impregnated with a platinum-rhodium catalyst, which catalyzes the conversion of nitric oxide into dinitrogen and oxygen as well as the conversion of carbon monoxide and hydrocarbons such as octane into carbon dioxide and water vapor:
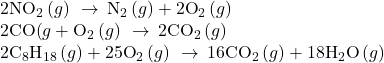
In order to be as efficient as possible, most catalytic converters are preheated by an electric heater. This ensures that the metals in the catalyst are fully active even before the automobile exhaust is hot enough to maintain appropriate reaction temperatures.
Enzyme Structure and Function – A Closer Look
The study of enzymes is an important interconnection between biology and chemistry. Enzymes are usually proteins (polypeptides) that help to control the rate of chemical reactions between biologically important compounds, particularly those that are involved in cellular metabolism. Different classes of enzymes perform a variety of functions, as shown in the Table 82-1 below.
| Table 82-1: Classes of Enzymes and Their Functions | |
|---|---|
| Class | Function |
| oxidoreductases | redox reactions |
| transferases | transfer of functional groups |
| hydrolases | hydrolysis reactions |
| lyases | group elimination to form double bonds |
| isomerases | isomerization |
| ligases | bond formation with ATP hydrolysis |
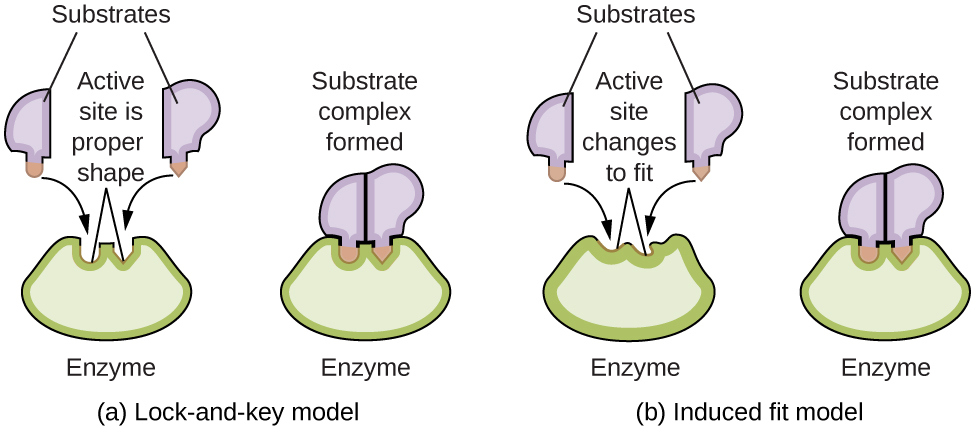
Enzyme molecules possess an active site, a part of the molecule with a shape that allows it to bond to a specific substrate (a reactant molecule), forming an enzyme-substrate complex as a reaction intermediate. Two of the many models that attempt to explain how this active site works are represented in Figure 82-8. The most simplistic model is referred to as the “lock-and-key hypothesis”, which suggests that the molecular shapes of the active site and substrate are complementary, fitting together like a key in a lock. The “induced fit hypothesis”, on the other hand, suggests that the enzyme molecule is flexible and changes shape to accommodate interaction with with the substrate. Both the lock-and-key model and the induced fit model account for the fact that enzymes can only bind with specific substrates, since in general a particular enzyme only catalyzes a particular reaction. However, the lock-and-key hypothesis does not account for the dramatic increase in rate effected by enzyme catalysts: perfect complementarity between the enzyme and substrate would serve to stabilize the substrate, preventing conversion to the transition state and, ultimately, products.
The Royal Society of Chemistry provides an excellent introduction to enzymes for students and teachers.
Key Concepts and Summary
Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. According to the Arrhenius Equation, a lower activation energy barrier will increase the rate constant, resulting in an increased rate of reaction. Catalysts can be homogenous (in the same phase as the reactants) or heterogeneous (a different phase than the reactants).
Section 82 Practice Problems
1. Account for the increase in reaction rate brought about by a catalyst.
Answer(s): The general mode of action for a catalyst is to provide a mechanism by which the reactants can unite more readily by taking a path with a lower reaction energy. The rates of both the forward and the reverse reactions are increased, leading to a faster achievement of equilibrium.
2. Compare the functions of homogeneous and heterogeneous catalysts.
3. Consider this scenario and answer the following questions: Chlorine atoms resulting from decomposition of chlorofluoromethanes, such as CCl2F2, catalyze the decomposition of ozone in the atmosphere. One simplified mechanism for the decomposition is:
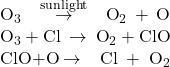
(a) Explain why chlorine atoms are catalysts in the gas-phase transformation:
![]()
(b) Nitric oxide is also involved in the decomposition of ozone by the mechanism:
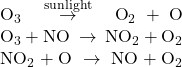
Is NO a catalyst for the decomposition? Explain your answer.
Answer(s): (a) Chlorine atoms are a catalyst because they react in the second step but are regenerated in the third step. Thus, they are not used up, which is a characteristic of catalysts. (b) NO is a catalyst for the same reason as in part (a).
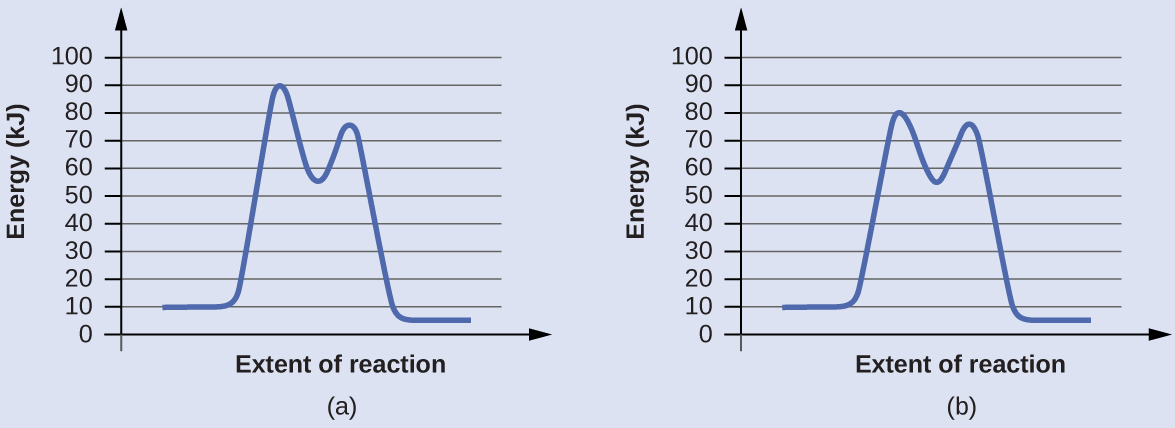
4. For each of the following pairs of reaction diagrams, identify which of the pair is catalyzed:
(a)
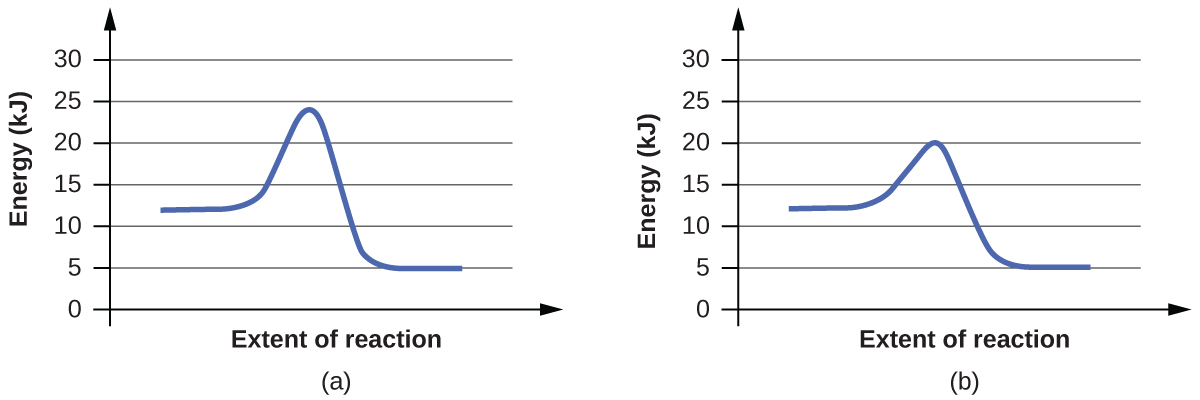
(b)
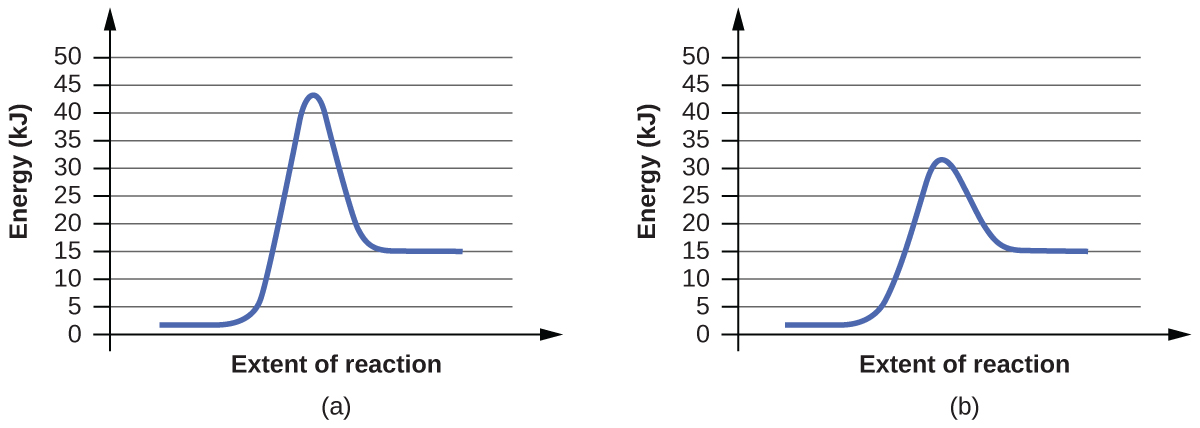
(c)
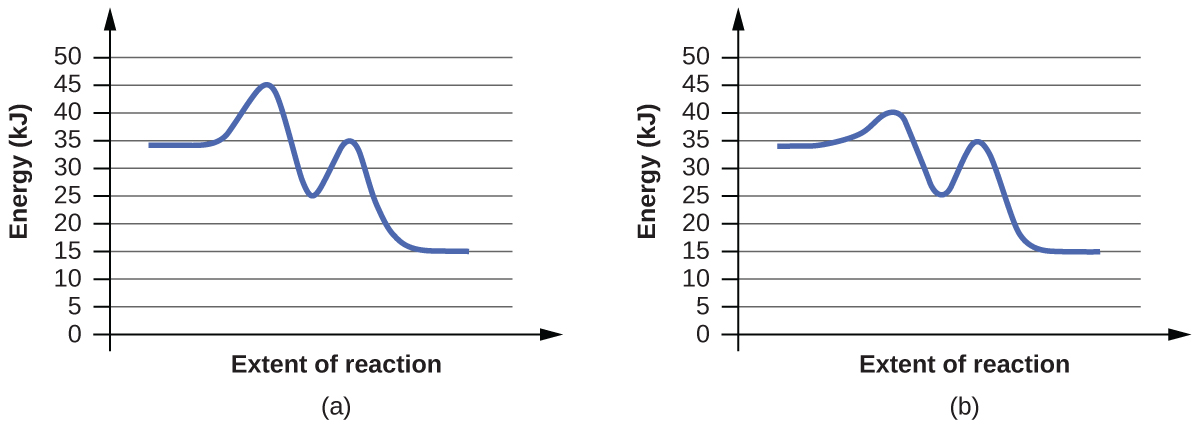
5. For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
(a)
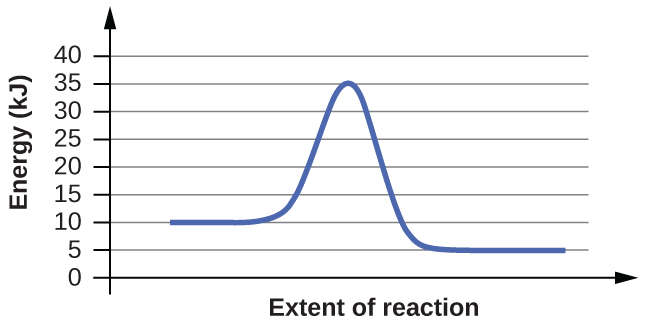
(b)
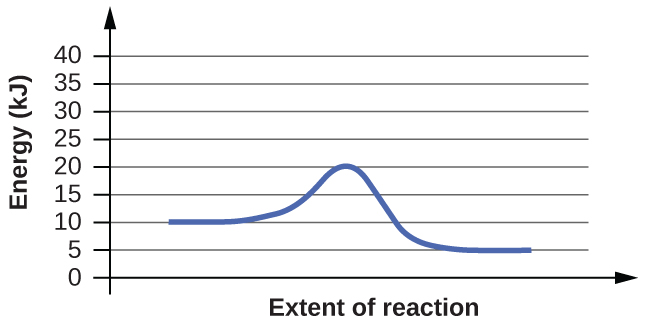
6. For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
(a)
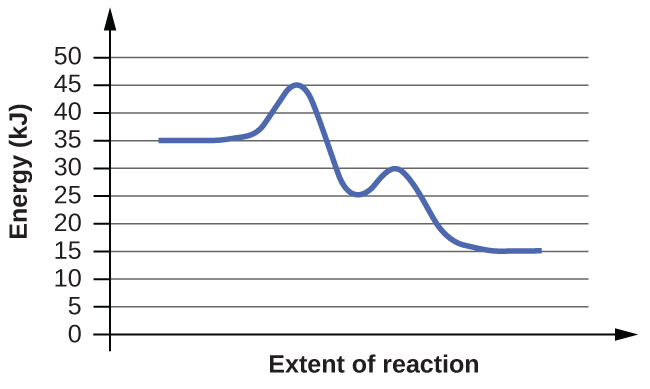
(b)
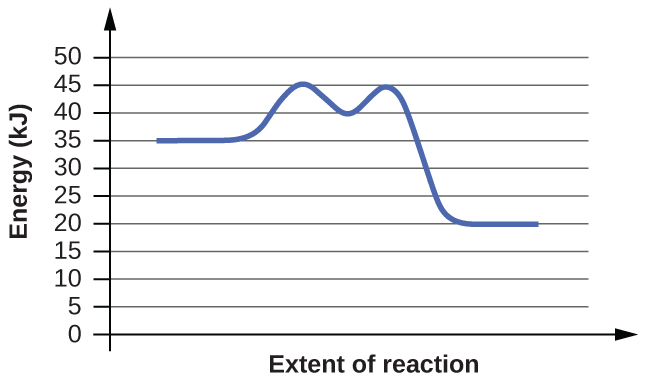
Answer(s): The energy needed to go from the initial state to the transition state is (a) 10 kJ; (b) 10 kJ.
Footnotes
- 1“The Nobel Prize in Chemistry 1995,” Nobel Prize.org, accessed February 18, 2015, http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1995/.
Glossary
- catalyst
- A species that increases the rate of reaction (in both the forward and reverse directions) by decreasing the activation energy barriers. A catalyst participates in a reaction but it is neither consumed nor produced (it is not part of the net reaction). A catalyst must be returned to its original condition by the end of the catalytic cycle, so that it can continue to participate in the reaction.
- heterogeneous catalyst
- catalyst present in a different phase from the reactants, furnishing a surface at which a reaction can occur
- homogeneous catalyst
- catalyst present in the same phase as the reactants

